The Solid State Class 12
Notes Chemistry Chapter 1
1. Solids are
substances which have fixed shape
and volume. 1’hey are characterised by rigidity, incompressibility, slow
diffusion and mechanical strength. They are classified as:
(a) Crystalline solids
(b) Amorphous solids .
2. The crystalline solids are further classified as:
(a) Metallic solids
(b) Ionic solids
(c) Covalent solids
(d) Molecular solids
3. A regular three dimensional arrangement of points in space is
called a space lattice or crystal lattice. There are only 14 three-dimensional
lattices known as Bravais lattices. The basic difference between the 14 Bravais
lattices are the angles between the faces and the relative proportion of the
sides.
4. A unit cell is the smallest unit of the crystal which when
repeated again and again gives the crystal of the given substance.
5. There are three types of unit cells based on the cube. These
are:
(a) Primitive or simple cube which has one constituent at each
comer.
(b) Body-centred cube in which one constituent at the centre of the
cube as well as one at each comer.
(c) Face-centred cube in which there is one constituent at the
centre of each face as well as one at each comer.
6. A pure metal in the solid crystalline state is composed of atoms
that are identical in shape and size. The identical spheres can be packed in a
number of ways.
7. The number of nearest neighbours of an atom, ion or a molecule
is called its coordination number.
8. In the hcp and ccp structures, about 74 percent of the available
space is occupied by the spheres. In bcc arrangement, about 68 percent of the
available space is filled up. In simple cubic structures, about 52.4 percent of
the available space is occupied by the spheres.
9. The density of the unit cell,
10. Any departure from perfectly ordered arrangement of atoms or
ions in crystals is called imperfection or defects. These are of two types:
(a) Point defects (b) Line defects
11. Schottky defects occurs when a pair of ions of opposite charge,
i.e., cations and anions are missing from the ideal lattice. The presence of a
large number of schottky defects lowers the density of a crystal, e.g., AgBr.
12. The atoms or ions which occupy the normally vacant interstitial
sites in a crystal are known as interstitials. It results in increase in density
of crystal.
13. Frenkel defects is a combination of schottky defects and
interstitials. It occurs when an ion leaves its position in the lattice and
occupies an interstitial site leaving a gap in the crystal.
14. Non-stoichiometric defects are a large number of inorganic
solids in which the ratio of the number of atoms of one kind to the number of
atoms of the other kind does not correspond to the ideal whole number ratio.
Such compounds are called non-stoichiometric compounds.
15. When there is an excess of metal ions in non- stoichiometric
compounds, the crystal lattice has vacant anion sites. These sites are occupied
by electrons. Hie anion sites occupied by electrons are called F-centres.
16. Based on their electrical conductivity, solids are classified
as:
(a) Conductors
(b) Insulators
(c) Semi conductors
17. Pure substances that show conducting behaviour like that of
silicon and germanium are called intrinsic semiconductors.
18. When solid substances are placed in a magnetic field, they do
not show the same behaviour. Depending on their response to magnetic field, the
substances are classified as:
(a) Diamagnetic substances:
(i) These substances are weakly repelled by a magnetic field.
(ii) The electrons are paired.
(b) Paramagnetic substances:
(i) These substances are weakly attracted by the magnetic field.
(ii) These substances have permanent magnetic dipoles due to die presence of
atoms, molecules or ions containing unpaired electrons.
19. Substances having unpaired electrons are classified as:
1. Solids
Solids have definite volume, shape, and mass due to the short distance between the fixed position of particles and strong interactions between them.
1.1 Characteristics Properties of the Solid State
(i) They have definite mass, volume and shape.
(ii) Intermolecular distances are short.
(iii) Intermolecular forces are strong.
(iv) Their constituent particles (atoms, molecules or ions) have fixed positions and can only oscillate about their mean positions.
(v) They are incompressible and rigid.
Students can refer to the short notes and MCQ questions along with separate solution pdf of this chapter for quick revision from the links below:
For More Information On Solid State, Watch The Below Videos:
1.2 Amorphous and Crystalline Solids
Solids can be classified as crystalline or amorphous on the basis of the nature of order present in the arrangement of their constituent particles. Amorphous solids behave like super cool liquids as the arrangement of constituent particles has short-range order, isotropic in nature and no sharp melting point. Crystalline solids have a characteristic shape, with the arrangement of constituent particles of long-range order, anisotropic in nature and a sharp melting point.
1.3 Classification of Crystalline Solids
The classification of crystalline solids is based on their property. The crystalline property depends on the nature of interactions between the constituent particles, and therefore these solids are divided into four different categories:
- Ionic solids
- Covalent or Network solids
- Molecular solids
- Metallic solids
1.4 Crystal Lattices and Unit Cells
Unit Cell
The smallest repeating unit of the crystal lattice is the unit cell, the building block of a crystal.
Types Of Unit Cell
A lattice can be generated by repeating a small portion called the unit cell. Below are some of the different varieties of the unit cell:
- Primitive Cubic Unit Cell
- Body-centered Cubic Unit Cell
- Face centered cubic unit cell

Crystal Lattices
A crystal structure is made of atoms. A crystal lattice is made of points. A crystal system is a set of axes. In other words, the structure is an ordered array of atoms, ions or molecules.
Characteristics of Crystal Lattice
(a) Each point in a lattice is called lattice point or lattice site.
(b) Each point in a crystal lattice represents one constituent particle which may be an atom, a molecule (a group of atoms) or an ion.
(c) Lattice points are joined by straight lines to bring out the geometry of the lattice.
1.5 Number of Atoms in a Unit Cell
1.5.1 Primitive Cubic unit Cell
The primitive cubic unit cell has atoms only at its corner. Each atom at a corner is shared between eight adjacent unit cells four unit cells in the same layer and four-unit cells of the upper or lower layer. Therefore, only 1/8th of an atom actually belongs to a particular unit cell.

1.5.2 Body-Centred Cubic unit Cell
A body-centred cubic unit cell has an atom at each of its corners and also one atom at its body centre.

Number of Atoms in BCC Cell:
Thus, in a BCC cell, we have:
- 8 corners × 1/8 per corner atom = 8 × 1/8 = 1 atom
- 1 body center atom = 1 × 1 = 1 atom
Therefore, the total number of atoms present per unit cell = 2 atoms.
1.5.3 Face-Centred Cubic unit Cell
A face-centred cubic unit cell contains atoms at all the corners and at the centre of all the faces of the cube. The atom present at the face-center is shared between 2 adjacent unit cells and only 1/2 of each atom belongs to an individual cell.

Number of Atoms in BCC Cell
a) 8 corners × 18 per corner atom = 8 × 18 = 1 atom
b) 6 face-centered atoms × 12 atom per unit cell = 3 atoms
Hence, the total number of atoms in a unit cell = 4 atoms
Thus, in a face-centered cubic unit cell, we have:
- 8 corners × 1/8 per corner atom = 8 × 1/8 = 1 atom
- 6 face-centered atoms × 1/2 atom per unit cell = 3 atoms
Therefore, the total number of atoms in a unit cell = 4 atoms.
1.6 Close Packed Structures
In solids, the constituent particles are close-packed, leaving the minimum vacant space.
(a) Close Packing in One Dimension
In close packing one dimension, spheres are arranged in a row such that adjacent atoms are in contact with each other. Coordination number is defined as the no. of nearest neighbour particles. In case of one dimension close packing, the coordination number is equal to two.
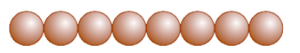
(b) Close packing in Two Dimensions
In two-dimensional close packing, a row of closed packed spheres is stacked to obtain a two-dimensional pattern.
his stacking is done in two ways:
Square close packing and Hexagonal close packing
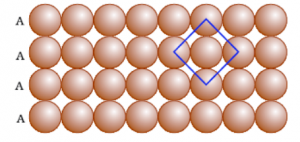
Packing in Solids: One and Two Dimensions
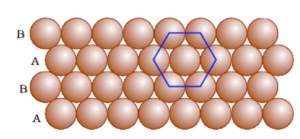
Hexagonal close-packing
(c) Close Packing in Three Dimensions

Crystalline solids exhibit a regular and repeating pattern of constituent particles. Three-dimensional closed packing are:
(i) Three-dimensional close packing forms two-dimensional square close-packed layers
(ii) Three-dimensional close packing from two-dimensional hexagonal close-packed layers.
1.6.1 Formula of a Compound and Number of Voids Filled
Voids literally mean gaps between the constituent particles. Voids in solid states mean the vacant space between the constituent particles in a closed packed structure.
There are two types of interstitial voids in a 3D structure:
Tetrahedral voids and Octahedral voids

Tetrahedral and octahedral void
1.7 Packing Efficiency
Packing Efficiency is the percentage of total space filled by the particles.
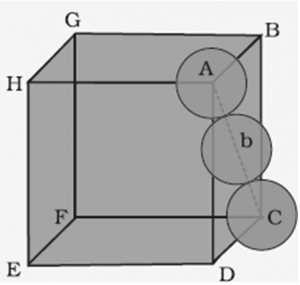
1.7.1 Packing Efficiency in hcp and ccp Structures
Hexagonal close packing (hcp) and cubic close packing (ccp) have the same packing efficiency.
Packing Efficiency of a Unit Cell
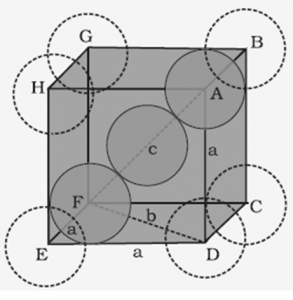
1.7.2 Efficiency Packing in Body-Centred Cubic Structures
In body centered cubic unit cell, one atom is located at body center apart from corners of the cube.
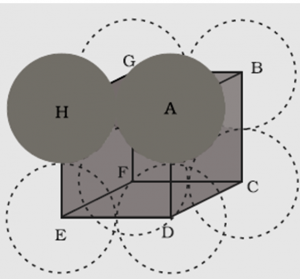
1.7.2 Packing Efficiency in Simple Cubic Lattice
In the simple cubic unit cell, atoms are located at the corners of the cube.
1.8 Calculations Involving Unit Cell Dimensions
The unit cell can be seen as a three-dimensional structure containing one or more atoms. We can determine the volume of this unit cell with the knowledge of the dimensions of the unit cell.
Mass of unit cell = number of atoms in unit cell × mass of each atom = z × m
Where, z = number of atoms in the unit cell, m = Mass of each atom
Mass of an atom can be given with the help of Avogadro number and molar mass as:
M/NA
Where M = molar mass
NA = Avogadro’s number
Volume of the unit cell, V = a3
=> Density of unit cell = mass of unit cell/ volume of the unit cell
=> Density of unit cell = m/V = z×ma/a3 = z×M/a3×NA
1.9 Imperfections in Solids
Point defects explain about the imperfections of solids along with the types of point defects. Point defects are accounted for when the crystallization process occurs at a very fast rate. These defects mainly happen due to deviation in the arrangement of constituting particles. The defects are of two types:
Point defects: Point defects are the irregularities or deviations from ideal arrangement around a point or an atom in a crystalline substance.
Line Defects: Line defects are the irregularities or deviations from an ideal arrangement in entire rows of lattice points. These irregularities are called crystal defects.
1.9.1 Types of Point Defects
Point defects can be classified into three types:
1. Stoichiometric defect – In this kind of point defect, the ratio of positive and negative ions (Stoichiometric) and electrical neutrality of a solid is not disturbed. Sometimes it is also known as intrinsic or thermodynamic defects. Fundamentally, they are of two types: Vacancy defect and Interstitial defect
2. Frenkel defect – In ionic solids generally, the smaller ion (cation) moves out of its place and occupies an intermolecular space. In this case, a vacancy defect is created on its original position and the interstitial defect is experienced at its new position.

3. Schottky defect – This kind of vacancy defects is found in Ionic Solids. But in ionic compounds, we need to balance the electrical neutrality of the compound so an equal number of anions and cations will be missing from the compound. It reduces the density of the substance. In this, the size of cations and anions are of almost the same.

1.10 Electrical Properties
Solids can be classified into three types on the basis of their conductivities. They are:
(i) Conductors
(ii) Insulators
(iii) Semiconductors
1.11 Magnetic Properties
To study the magnetic properties of Magnetic Materials, the material is usually placed in a uniform magnetic field and then the magnetic field is varied. There are five major kinds of magnetic behaviour:
(i) Diamagnetic materials
(ii) Paramagnetic materials
(iii) Ferromagnetic materials
(iv) Antiferromagnetic materials
(v) Ferrimagnetic materials
Few Important Questions
- Explain the term coordination number.
- Distinguish between Cubic close-packing and Hexagonal close-packing.
- Explain why Ionic solids are brittle and hard.
- Distinguish between a semiconductor and a conductor.
- Explain Paramagnetism with suitable example.
DR OMAR CLASSES MOB.-9450149685
(LIG 120, BARRA 3, KANPUR)
SOLUTION
Solution is a homogeneous mixture of two or more substances in same or different physical phases. The substances forming the solution are called components of the solution. On the basis of number of components a solution of two components is called binary solution.
Solute and Solvent
In a binary solution, solvent is the component which is present in large quantity while the other component is known as solute.
Classification of Solutions
(A) Following types of solutions are seen on the basis of physical state of solute and solvent.
(B) Depending upon the amount of solute dissolved in a solvent we have the following types of solutions:
(i) Unsaturated solution A solution in which more solute can be dissolved without raising temperature is called an unsaturated solution.
(ii) Saturated solution A solution in which no solute can be dissolved further at a given temperature is called a saturated solution.
(iii) Supersaturated solution A solution which contains more solute than that would be necessary to saturate it at a given temperature is called a supersaturated solution.
Solubility
DR OMAR CLASSES MOB.-9450149685
(LIG 120, BARRA 3, KANPUR)
The maximum amount of a solute that can be dissolved in a given amount of solvent (generally 100 g) at a given temperature is termed as its solubility at that temperature.
The solubility of a solute in a liquid depends upon the following factors:
(i) Nature of the solute
(ii) Nature of the solvent
(iii) Temperature of the solution
(iv) Pressure (in case of gases)
Henry’s Law
The most commonly used form of Henry’s law states “the partial pressure (P) of the gas in vapour phase is proportional to the mole fraction (x) of the gas in the solution” and is expressed as
p = KH . x
Greater the value of KH, higher the solubility of the gas. The value of KH decreases with increase in the temperature. Thus, aquatic species are more comfortable in cold water [more dissolved O2] rather than Warm water.
Applications
1. In manufacture of soft drinks and soda water, CO2 is passed at high pressure to increase its solubility.
2. To minimise the painful effects (bends) accompanying the decompression of deep sea divers. O2 diluted with less soluble. He gas is used as breathing gas.
3. At high altitudes, the partial pressure of O2 is less then that at the ground level. This leads to low concentrations of O2 in the blood of climbers which causes ‘anoxia’.
Concentration of Solutions
The concentration of a solution is defined as the relative amount of solute present in a solution. On the basis of concentration of solution there are two types of solutions.
(i) Dilute solution
(ii) Concentrated solution
Methods of Expressing Concentration of Solutions
Various expression for the concentrations of solutions can be summarised as
(i) Percentage by weight (w / w %) It is defined as the amount of solute present in 100 g of solution.
w / w % = weight of solute / weight of solution * 100
(ii) Percentage by volume (w / V%) It is defined as the weight 01 solute present in 100 mL of solution.
w / V % = weight of solute / weight of solution * 100
DR OMAR CLASSES MOB.-9450149685
(LIG 120, BARRA 3, KANPUR)
or the volume of solute present in 100 mL of solution.
u / V % = volume of solute / volume of solution * 100
(iii) Mole fraction (x) It is defined as the ratio of the number of moles of a component to the total number of moles of all the components. For a binary solution, if the number of moles of A and B are nA and nB respectively, the mole fraction of A will be
(iv) Parts per million (ppm) It is defined as the parts of a component per million parts (106) of the solution. It is widely used when a solute is present in trace quantities.
ppm = number of parts of the component / total number of parts of all the components * 106
(v) Molarity (M) It is the number of moles of solute present in 1L(dm3) of the solution.
M = number of moles of solute / volume of solution (L)
M = mass of solute (in gram) * 1000 / mol. wt. of solute x volume of solution (in mL)
Molarity varies with temperature due to change in volume of solution.
[When molarity of a solution is 1 M, it is called a molar solution. 0.1 M solution is called a decimolar solution while 0.5 M solution is known as semi molar solution]
Molarity = Percent by mass * density * 10 / molecular weight
Dilution law, M1 V1 = M2 V2 (for dilution from volume V1 to V2)
For reaction between two reactants, M1 V1 / n1 = M2 V2 / n2
(vi) Molality (m) It is the number of moles of solute per kilogram of the solvent.
Molality = mass of solute in gram * 1000 / mol. wt. of solute * mass of solvent (in g)
Molality is independent of temperature.
[Whcn solvent used is water, a molar (1 M) solution is more concentrated than a molal (1 M) solution.]
(vii) Normality (N) The number of gram equivalents of solute present in 1 L of solution.
Normality = number of grams – equivalent of solute / volume of solution in L
Number of gram-equivalents of solute = mass of solute in gram / equivalent weight
DR OMAR CLASSES MOB.-9450149685
(LIG 120, BARRA 3, KANPUR)
[Relationship between normality and molarity N x Eq. weight = M x mol. weight ]
If two solutions of the same solute having volumes and molarities V1, M1 and V2, M2 are mixed, the molarity of the resulting solution is
To dilute V1 mL of a solution having molarity M1 to molarity M2 up to the final volume V2 mL, the volume of water added is
(viii) Formality (F) It is the number of formula weights of solute present per litre of the solution.
Formality = moles of substance added to solution / volume of solution (in L))
(ix) Mass fraction Mass fraction of any component in the solution is the mass of that component divided by the total mass of the solution.
Molality, mole fraction and mass fraction are preferred over molarity, normality, etc., because former involve weights which do not change with temperature.
(x) Demal (D) It represents one mole of solute present in 1L of solution at O°C.
Raoult’s Law
The Raoult’s law states “For a solution of two volatile liquids, the vapour pressure of each liquid in the solution is less than the respective vapour pressure of the pure liquids and the equilibrium partial vapour pressure of the liquid is directly proportional to its mole fraction.
For a solution containing two liquids A and B, the partial vapour pressure of liquid A is
The proportionality constant is obtained by considering the pure liquid when χA = 1 then k = P°A, the vapour pressure of pure liquid, hence
DR OMAR CLASSES MOB.-9450149685
(LIG 120, BARRA 3, KANPUR)
Konowaloff Rule
At any fixed temperature, the vapour phase is always richer in the more volatile component as compared to the solution phase. In other words, mole fraction of the more volatile component is always greater in the vapour phase than in the solution phase.
The composition of vapour phase in equilibrium with the solution is determined by the partial pressure of components. If Y1 and Y2 are the
component 1 and 2 respectively in the vapour phase then. using Dalton’s law of partial pressure,
p1 = y1 * Ptotal
p2 = y2 * Ptotal
Ideal Solutions
Those solutions in which solute-solute (B-B) and solvent-solvent (A-A) interactions are almost similar to solvent solute (A-B) interactions are called ideal solutions. These solutions satisfy the following conditions :
(i) Solution must obey Raoult’s law, i.e.,
(ii) ΔHmix = 0 (No energy evolved or absorbed)
(iii) ΔVmix = 0 (No expansion or contraction on mixing)
DR OMAR CLASSES MOB.-9450149685
(LIG 120, BARRA 3, KANPUR)
Some solutions behave like nearly ideal solutions, e.g., benzene + toluene. n-hexane + n-heptane, ethyl iodide + ethyl bromide, chlorobenzene + bromobenzene.
Non-ideal Solutions
Those solutions which shows deviation from Raoult’s law is called non-ideal solution.
For such solutions,
ΔHmix ≠ 0
ΔVmix ≠ 0
(a) Non-ideal solutions showing positive deviation In such a case, the A – B interactions are weaker than A – A or B – B interactions and the observed vapour pressure of each component and the total vapour pressure are greater than that predicted by Raoult’s law.
For such solutions
(b) Non-ideal solution showing negative deviation In such a case, the A – B interactions are stronger than A – A or B – B interactions and the observed vapour pressure of each component and the total vapour pressure are lesser than that predicted by Raoult’s law.
DR OMAR CLASSES MOB.-9450149685
(LIG 120, BARRA 3, KANPUR)
Azeotropic Mixture
A mixture of two liquids which boils at a particular temperature like a pure liquid and distils over in the same composition is known as constant boiling mixtures. These are formed by non-ideal solutions.
(i) Minimum boiling azeotropes are formed by those liquid pairs which show positive deviation from ideal behaviour. Such azeotropes have boiling points lower than either of the components, e.g., C2H5OH (95.57%) + H2O (4.43%)(by mass).
(ii) Maximum boiling azeotropes are formed by those liquid pain; which show negative deviation from ideal behaviour. Such azeotropes have boiling points higher than either of the components. e.g., H2O(20.22O%)+ HCl (79.78%] by mass.
Colligative Properties
[Colligatil1e : from Latin. = Co mean ‘together’; ligare means ‘to bind’.]
Colligative properties are those properties which depends only upon the number of solute particles in a solution irrespective of their nature.
Relative Lowering of Vapour Pressure
It is the ratio of lowering in vapour pressure to vapour pressure of pure solvent. The relative lowering in vapour pressure of solution containing a nonvolatile solute is equal to the mole fraction of solute in the solution.
Above expression is used to find the molecular weight of an unknown solute dissolved in a given solvent. Where, WB and WA = mass of Solute and solvent respectively. MB and MA = molecular weight of solute and solvent respectively.
Ostwald and Walker method is used to determine the relative lowering of vapour pressure.
Elevation in Boiling Point (ΔTb)
Boiling point of a liquid is the temperature at which its vapour pressure becomes equal to the atmospheric pressure. As the vapour pressure of a solution containing a nonvolatile solute is lower than that of the pure solvent, it boiling point will be higher than that of the pure solvent as shown in figure. The increase in boiling point is known as elevation in boiling point, ΔTb
ΔTb = Tb – T°b
ΔTb = Kb m (where; m = molality)
Kb is molal elevation constant or ebullioscopic constant. Molecular mass of solute can be calculated as
where, WB and WA = mass of solute and solvent respectively.
Kb has units of K / m or K kg mol-1, for water, Kb = 0.52 K kg mol-1
The boiling point elevation of a solution is determined by
(i) Landsberger’s method
(ii) Cottrell’s method
Depression in Freezing Point (ΔTf)
Freezing point of a liquid is the temperature at which vapour pressure of the solvent in its liquid and solid phase become equal. As we know that vapour pressure of solution containing non-volatile solute is lower than that of pure solvent, solid form gets separated out at a lower temperature as shown in the figure.
This decrease in freezing point of a liquid is known as depression in freezing point.
Depression in freezing point (ΔTf) = T°f – Tf
To find molecular mass of solute,
where, Kf is molal depression constant or cryoscopic constant.
Kf has units of K / m or K kg mol-1.
Ethylene glycol is usually added to water in the radiator to lower its freezing point. It is called antifreeze solution.
[Common salt (NaCI) and anhydrous CaC12 are used to clear snow on the roads because they depress the freezing point of water. The freezing point depression is determined by Beckmann method or Rast method.]
Calculations of molal elevation constant (Kb) and molal depression constant (Kf)
Osmotic Pressure (π)
Osmosis is the phenomenon of spontaneous flow of the solvent molecules through a semipermeable membrane from pure solvent to solution or from a dilute solution to concentrated solution. It was first observed by Abbe Nollet.
Some natural semipermeable membranes are animal bladder, cell membrane etc.
CU2[Fe(CN)6]is an artificial semipermeable membrane which does not work in non-aqueous solutions as it dissolves in them.
Osmosis may be
(i) Exosmosis It is outward flow of water or solvent from a cell through semipermeable membrane.
(ii) Endosmosis It is inward flow of water or solvent from a cell through a semipermeable membrane.
The hydrostatic pressure developed on the solution which just prevents the osmosis of pure solvent into the solution through a semipermeable membrane is called osmotic pressure.
where, d = density, R = solution constant,
T = temperature, MB = molar mass of solute
Osmotic pressure can be determined by anyone of the method listed below
(i) Pfeffer’s method
(ii) Berkeley and Hartley’s method (very good method)
(iii) Morse and Frazer’s method
On the basis of osmotic pressure, -the solution can be
(i) Hypertonic solution A solution is called hypertonic if its osmotic pressure is higher than that of the solution from which it is separated by a semipermeable membrane.
When a plant cell is placed in a hypertonic solution, the fluid from the plant cell comes out and cell shrinks, this phenomenon is called plasmolysis.
(ii) Hypotonic solution A solution is called hypotonic if its osmotic pressure is lower than that of the solution from which it is separated by a semipermeable membrane.
(iii) Isotonic solution Two solutions are called isotonic if they exert the same osmotic pressure. These solutions have same molar concentration. 0.91% solution of pure NaCl is isotonic with human RBC’s.
Two solutions are isotonic if they have the same molar concentration, e.g., if x % solution of X is isotonic with y % solution of Y, this means molar concentration of X = Molar concentration of Y
Osmotic pressure method is the best method for determining the molecular masses of polymers since observed value of any other colligative property is too small to be measured with reasonable accuracy.
Reverse osmosis When the external pressure applied on the solution is more than osmotic pressure, the solvent flows from the solution to the pure solvent, I which is called reverse osmosis. Desalination of sea water is done by reverse Osmosis.
Abnormal Molecular Masses
In some cases, observed colligative properties deviate from their normal calculated values due to association or dissociation of molecules. As we know,
Colligative property ∝ 1 / MB
lienee, higher and lower values of molar mass is observed in case of association and dissociation respectively, e.g., in benzene, acetic acid gets associated, so, its observed molecular mass is 120. Similarly KCI undergoes dissociation in aqueous solution, so its observed molecular mass is 37.25.
These observed values are corrected by multiplying with van’t Hoff factor (i).
van’t Hoff Factor (i)
It is the ratio of observed value of colligative property to the calculated value of colligative property.
i = observed value of colligative property / calculated value of colligative property
or i = normal molecular mass / observed molecular mass
or i = number of particles after association or dissociation / number of particles initially
So to correct the observed value of molar mass, van’t Hoff factor (i) must be included in different expressions for colligative properties.
Degree of Dissociation (α) and van’t Hoff Factor (i)
(i) If one molecule of a substance gets dissociated into n particles or molecules and α is the degree of dissociation then
Degree of Association (α) and van’t Hoff Factor (i)
If n molecules of a substance A associate to form An and α is the degree of association then
DR OMAR CLASSES MOB.-9450149685
(LIG 120, BARRA 3, KANPUR)
DR OMAR CLASSES MOB.-9450149685
(LIG 120, BARRA 3, KANPUR)
van’t Hoff factor (i) > 1 for solutes undergoing dissociation and it is < 1 for solutes undergoing association.
NCERT TEXTBOOK QUESTIONS SOLVED
2.1. Calculate the mass percentage of benzene (C6H6) and carbon tetrachloride (CCl4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride.
Ans: Mass of solution = Mass of C6H6 + Mass of CCl4
= 22 g+122 g= 144 g
Mass % of benzene = 22/144 x 100 =15.28 %
Mass % of CCl4 = 122/144 x 100 = 84.72 %
2.2. Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
Ans: 30% by mass of C6H6 in CCl4 => 30 g C6H6 in 100 g solution
no. of moles of C6H6,(nC6h6) = 30/78 = 0.385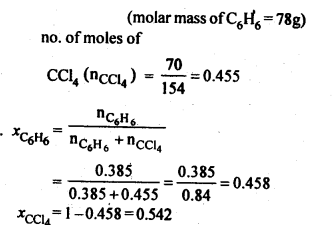
2.3. Calculate the molarity of each of the following solutions
(a) 30 g of Co(NO3)26H2O in 4·3 L of solution
(b) 30 mL of 0-5 M H2SO4 diluted to 500 mL.
Ans: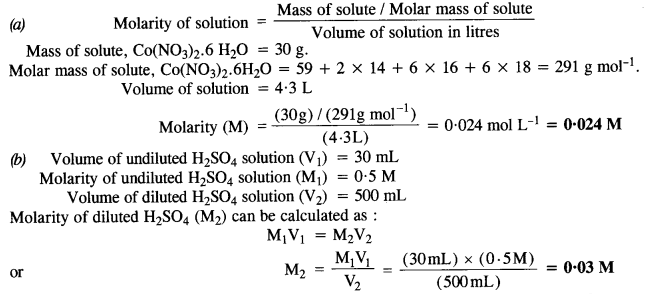
2.4. Calculate the mass of urea (NH2CONH2) required in making 2.5 kg of 0.25 molal aqueous solution.
Ans: 0.25 Molal aqueous solution to urea means that
moles of urea = 0.25 mole
mass of solvent (NH2CONH2) = 60 g mol-1
.’. 0.25 mole of urea = 0.25 x 60=15g
Mass of solution = 1000+15 = 1015g = 1.015 kg
1.015 kg of urea solution contains 15g of urea
.’. 2.5 kg of solution contains urea =15/1.015 x 2.5 = 37 g
2.5. Calculate
(a) molality
(b) molarity and
(c) mole fraction of KI if the density of 20% (mass/mass) aqueous KI solution is 1·202 g mL-1.
Ans:
Step I. Calculation of molality of solution
Weight of KI in 100 g of the solution = 20 g
Weight of water in the solution = 100 – 20 = 80 g = 0-08 kg
Molar mass of KI = 39 + 127 = 166 g mol-1.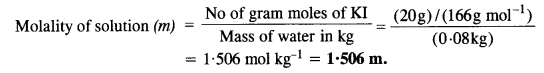
Step II. Calculation of molarity of solution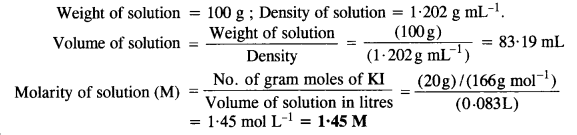
Step III. Calculation of mole fraction of Kl
2.6. H2 S, a toxic gas with rotten egg like smell, is used for the qualitative analysis. If the solubility of H2S in water at STP is 0.195 m, calculate Henry’s law constant.
Ans: Solubility of H2S gas = 0.195 m
= 0.195 mole in 1 kg of solvent
1 kg of solvent = 1000g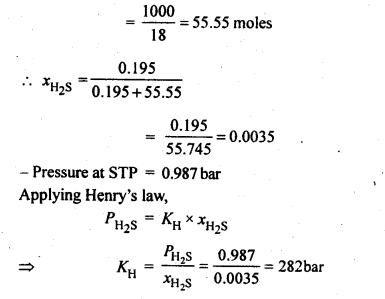
2.7. Henry’s law constant for CO2 in water is 1.67 x 108 Pa at 298 K. Calculate the quantity of CO2 in 500 mL of soda water when packed under 2.5 atm CO2 pressure at 298 K.
Ans.: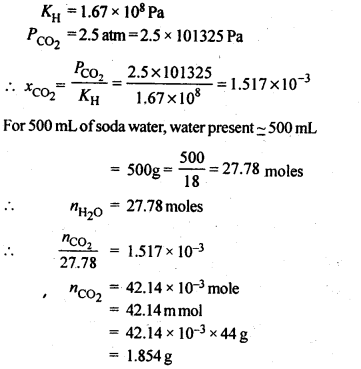
2.8 The vapour pressures of pure liquids A and B are 450 mm and 700 mm of Hg respectively at 350 K. Calculate the composition of the liquid mixture if total vapour pressure is 600 mm of Hg. Also find the composition in the vapour phase.
Ans:
Vapour pressure of pure liquid A (
Vapour pressure of pure liquid B (
Total vapour pressure of the solution (P) = 600 mm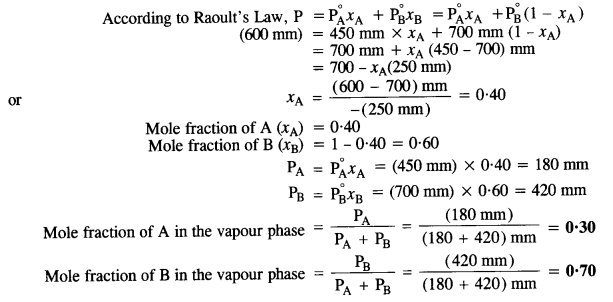
.9. Vapour pressure of pure water at 298 K is 23.8 m m Hg. 50 g of urea (NH2CONH2) is dissolved in 850 g of water. Calculate the vapour pressure of water for this solution and its relative lowering.
Ans: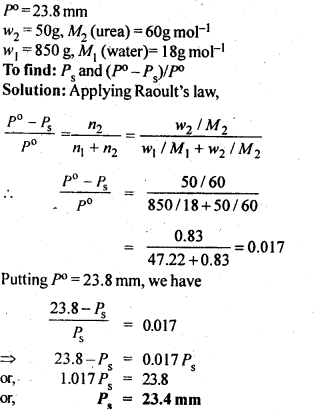
2.10. Boiling point of water at 750 mm Hg is 99.63°C. How much sucrose is to be added to 500 g of water such that it boils at 100°C.
Ans: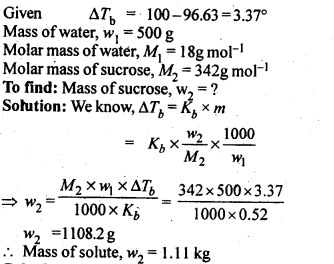
2.11 Calculate the mass of ascorbic acid (vitamin C, C6H8O6) to be dissolved in 75 g of acetic acid to lower its melting point by 1·5°C. (Kf for CH3COOH) = 3·9 K kg mol-1)
Ans: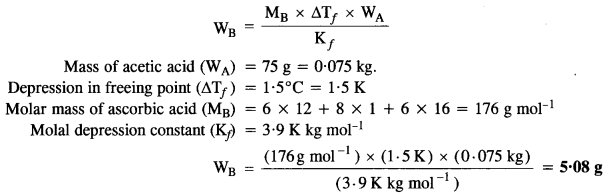
2.12. Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water at 37°C.
Ans: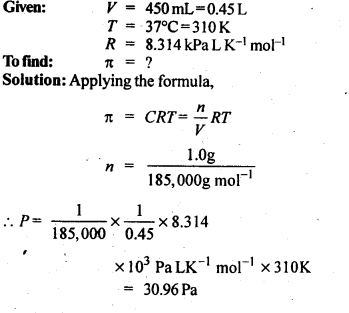
NCERT EXERCISES
2.1. Define the terra solution. How many types of solutions are formed? Write briefly about each type with an example.
Sol: A solution is a homogeneous mixture of two or more chemically non-reacting substances. Types of solutions: There are nine types of solutions.
Types of Solution Examples
Gaseous solutions
(a) Gas in gas Air, mixture of 02 and N2, etc.
(b) Liquid in gas Water vapour
(c) Solid in gas Camphor vapours in N2 gas, smoke etc.
Liquid solutions
(a) Gas in liquid C02 dissolved in water (aerated water), and 02 dissolved in water, etc.
(b) Liquid in liquid Ethanol dissolved in water, etc.
(c) Solid in liquid Sugar dissolved in water, saline water, etc.
Solid solutions
(a) Gas in solid Solution of hydrogen in palladium
(b) Liquid in solid Amalgams, e.g., Na-Hg
(c) Solid in solid Gold ornaments (Cu/Ag with Au)
2.2. Suppose a solid solution is formed between two substances, one whose particles are very large and the other whose particles are very small. What type of solid solution is this likely to be ?
Sol: The solution likely to be formed is interstitial solid solution.
2.3 Define the following terms:
(i) Mole fraction
(ii) Molality
(iii) Molarity
(iv) Mass percentage
Sol: (i) Mole fraction: It is defined as the ratio of the number of moles of the solute to the total number of moles in the solution. If A is the number of moles of solute dissolved in B moles of solvent, then Mole fraction of solute
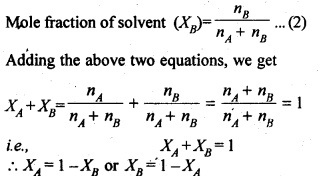
(ii) Molality: It is defined as die number of moles of a solute present in 1000g (1kg) of a solvent.![]()
NOTE: Molality is considered better way of expressing concentration of solutions, as compared to molarity because molality does not change with change in temperature since the mass of solvent does not vary with temperature,
(iii) Molarity: It is defined as the number of moles of solute present in one litre of solution.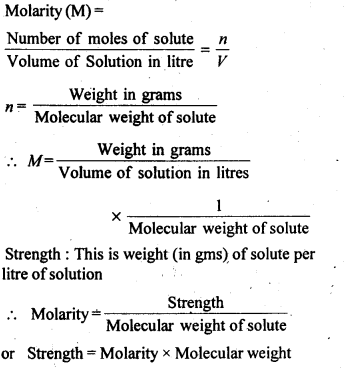
NOTE: Molarity is the most common way of expressing concentration of a solution in laboratory. However, it has one disadvantage. It changes with temperature because volume of a solution alters due to expansion and contraction of the liquid with temperature.
(iv) Mass percentage: It is the amount of solute in grams present in 100g of solution.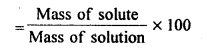
2.4. Concentrated nitric acid used in the laboratory work is 68% nitric acid by mass in aqueous solution. What should be the molarity of such a sample of acid if the density of the solution is 1·504 g mL-1 ?
Sol: Mass of HNO3 in solution = 68 g
Molar mass of HNO3 = 63 g mol-1
Mass of solution = 100 g
Density of solution = 1·504 g mL-1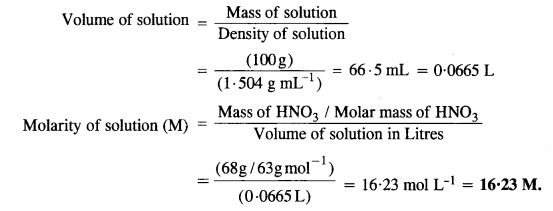
2.5. A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of solution is 1 .2 g m L-1, then what shall be the molarity of the solution?
Sol: 10 percent w/w solution of glucose in water means 10g glucose and 90g of water.
Molar mass of glucose = 180g mol-1 and molar mass of water = 18g mol-1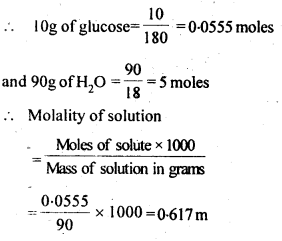

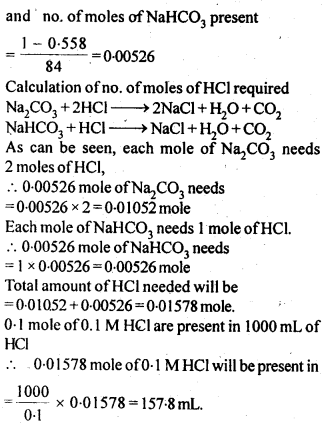
2.6. How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2C03 and NaHCO3 containing equimolar amounts of both?
Sol: Calculation of no. of moles of components in the mixture.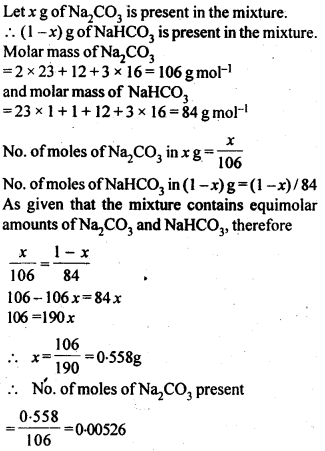
2.7. Calculate the percentage composition in terms of mass of a solution obtained by mixing 300 g of a 25% and 400 g of a 40% solution by mass.
Sol: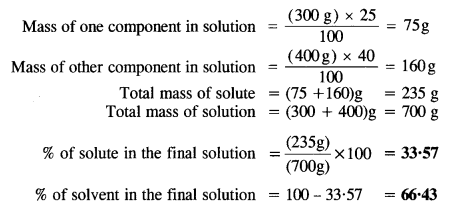
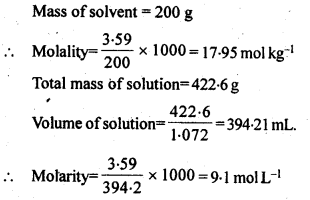
2.8. An antifreeze solution is prepared from 222.6 g of ethylene glycol, (C2 H6O2 ) and200 g of water. Calculate the molality of the solution. If the density of the solution is 1.072 g mL-1, then what shall be the molarity of the solution?
Sol: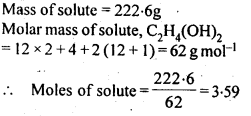
2.9. A sample of drinking water was found to be severely contaminated with chloroform (CHCl3), supposed to be a carcinogen. The level of contamination was 15 ppm (by mass).
(i) express this in percent by mass.
(ii) determine the molality of chloroform in the water sample.
Sol: 15 ppm means 15 parts in million (106) by mass in the solution.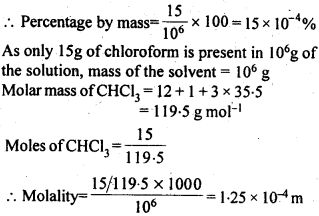
2.10. What role does the molecular interaction play in solution of alcohol in water?
Sol: In case of alcohol as well as water, the molecules are interlinked by intermolecular hydrogen bonding. However, the hydrogen bonding is also present in the molecules of alcohol and water in the solution but it is comparatively less than both alcohol and water. As a result, the magnitude of attractive forces tends to decrease and the solution shows positive deviation from Raoult’s Law. This will lead to increase in vapour pressure of the solution and also decrease in its boiling point.
2.11. Why do gases always tend to be less soluble in liquids as the temperature is raised?
Sol: When gases are dissolved in water, it is accompanied by a release of heat energy, i.e., process is exothermic. When the temperature is increased, according to Lechatlier’s Principle, the equilibrium shifts in backward direction, and thus gases becomes less soluble in liquids.
2.12. State Henry’s law and mention some of its important applications.
Sol:
Henry’s law: The solubility of a gas in a liquid at a particular temperature is directly proportional to the pressure of the gas in equilibrium with the liquid at that temperature.
or
The partial pressure of a gas in vapour phase is proportional to the mole fraction of the gas (x) in the solution. p = KHX
where KH is Henry’s law constant.
Applications of Henry’s law :
(i) In order to increase the solubility of CO2 gas in soft drinks and soda water, the bottles are normally sealed under high pressure. Increase in pressure increases the solubility of a gas in a solvent according to Henry’s Law. If the bottle is opened by removing the stopper or seal, the pressure on the surface of the gas will suddenly decrease. This will cause a decrease in the solubility of the gas in the liquid i.e. water. As a result, it will rush out of the bottle producing a hissing noise or with a fiz.
(ii) As pointed above, oxygen to be used by deep sea divers is generally diluted with helium inorder to reduce or minimise the painfril effects during decompression.
(iii) As the partial pressure of oxygen in air is high, in lungs it combines with haemoglobin to form oxyhaemoglobin. In tissues, the partial pressure of oxygen is comparatively low. Therefore, oxyhaemoglobin releases oxygen in order to carry out cellular activities.
2.13. The partial pressure of ethane over a solution containing 6.56 × 10-3 g of ethane is 1 bar. If the solution contains 5.00 × 10-2 g of ethane, then what shall be the partial pressure of the gas?
Sol: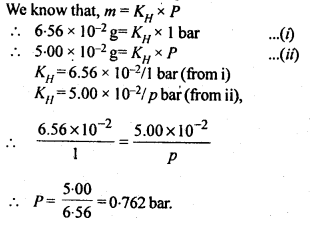
2.14. According to Raoult’s law, what is meant by positive and negative deviaitions and how is the sign of ∆solH related to positive and negative deviations from Raoult’s law?
Sol: Solutions having vapour pressures more than that expected from Raoult’s law are said to exhibit positive deviation. In these solutions solvent – solute interactions are weaker and ∆solH is positive because stronger A – A or B – B interactions are replaced by weaker A – B interactions. Breaking of the stronger interactions requires more energy & less energy is released on formation of weaker interactions. So overall ∆sol H is positive. Similarly ∆solV is positive i.e. the volume of solution is some what more than sum of volumes of solvent and solute.
So there is expansion in volume on solution formation.
Similarly in case of solutions exhibiting negative deviations, A – B interactions are stronger than A-A&B-B. So weaker interactions are replaced by stronger interactions so , there is release of energy i.e. ∆sol H is negative.
2.15. An aqueous solution of 2 percent non-volatile solute exerts a pressure of 1·004 bar at the boiling point of the solvent. What is the molecular mass of the solute ?
Sol:
According to Raoult’s Law,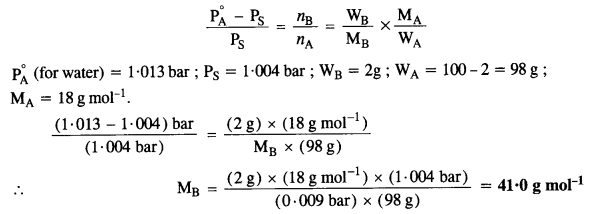
2.16 Heptane and octane form an ideal solution. At 373 K, the vapour pressures of the two liquid components are 105.2 kPa and 46.8 kPa respectively. What will be the vapour pressure of a mixture of 26.0 g of heptane and 35.0 g of octane?
Sol.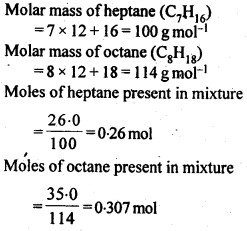
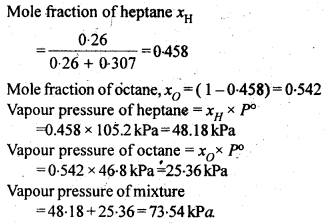
2.17. The vapour pressure of water is 12.3 kPa at 300 K. Calculate vapour pressure of 1 molal solution of a non-volatile solute in it
Sol: 1 molal solution of solute means 1 mole of solute in 1000g of the solvent.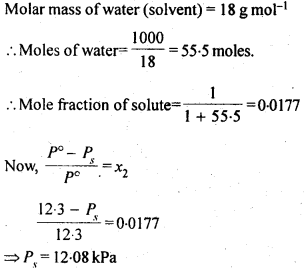
2.18. Calculate the mass of a non-volatile solute (molecular mass 40 g mol-1) that should be dissolved in 114 g of octane to reduce its pressure to 80%. (C.B.S.E. Outside Delhi 2008)
Sol: According to Raoult’s Law,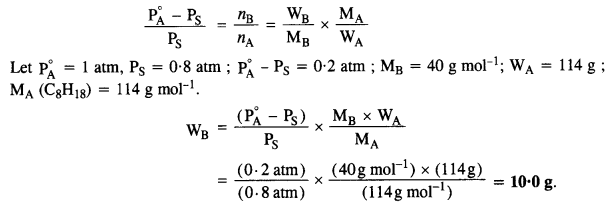
2.19. A solution containing 30g of non-volatile solute exactly in 90 g of water has a vapour pressure of 2.8 kPa at 298 K. Further, 18g of water is then added to the solution and the new of vapour pressure becomes 2.9 kPa at 298 K. Calculate
(i) molar mass of the solute.
(ii) vapour pressure of water at 298 K.
Sol: Let the molar mass of solute = Mg mol-1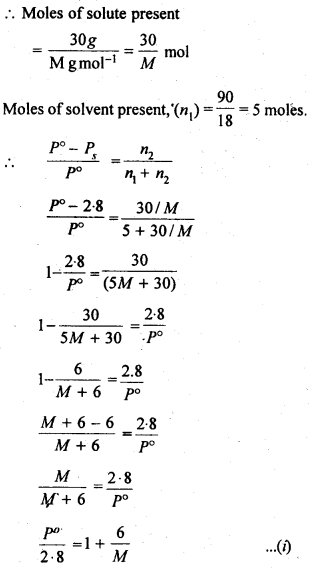
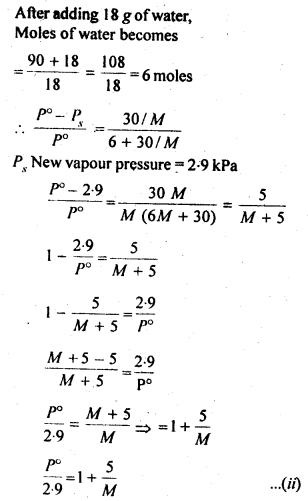
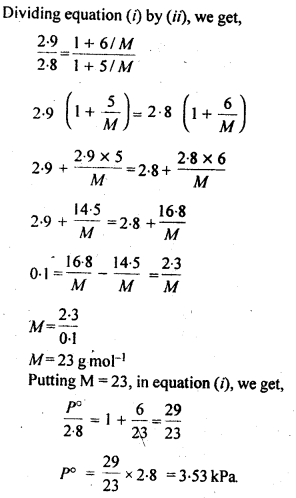
2.20. A 5% solution (by mass) of cane sugar in water has freezing point of 271 K. Calculate the freezing point of 5% glucose in water if freezing point of pure water is 273.15 K.
Sol: Mass of sugar in 5% (by mass) solution means 5gin 100g of solvent (water)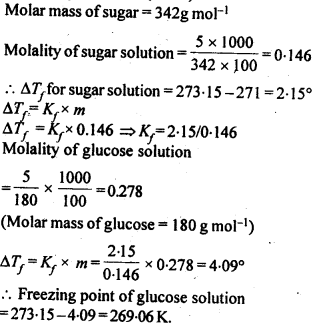
2.21. Two elements A and B form compounds having formula AB2 and AB4. When dissolved in 20g of benzene (C6H6), 1 g of AB2 lowers the freezing point by 2.3 K whereas 1.0 g of AB4 lowers it by 1.3 K. The molar depression constant for benzene is 5.1 K kg mol-1. Calculate atomic masses of A and B.
Sol: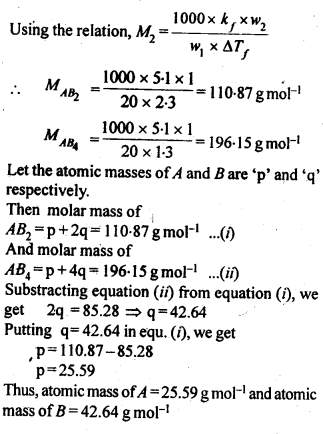
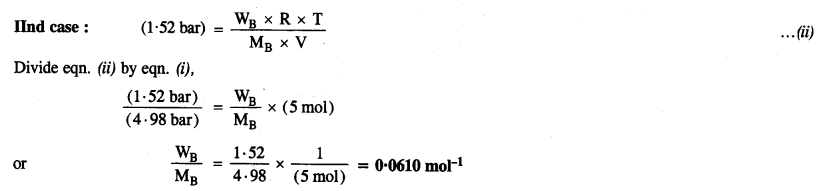
2.22. At 300 K, 36 g glucose present per litre in its solution has osmotic pressure of 4·98 bar. If the osmotic pressure of the solution is 1·52 bar at the same temperature, what would be its concentration?
Sol: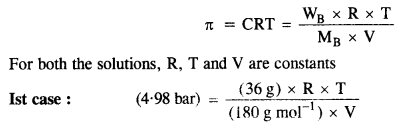
2.23. Suggest the most important type of intermolecular attractive interaction in the following pairs:
(i) n-hexane and n-octane
(ii) I2 and CCl4.
(iii) NaCl04 and water
(iv) methanol and acetone
(v) acetonitrile (CH3CN) and acetone (C3H60)
Sol: (i) Both w-hexane and n-octane are non-polar. Thus, the intermolecular interactions will be London dispersion forces.
(ii) Both I2 and CCl4 are non-polar. Thus, the intermolecular interactions will be London dispersion forces.
(iii) NaCl04 is an ionic compound and gives Na+ and Cl04– ions in the Solution. Water is a polar molecule. Thus, the intermolecular interactions will be ion-dipole interactions.
(iv) Both methanol and acetone are polar molecules. Thus, intermolecular interactions will be dipole-dipole interactions.
(v) Both CH3CN and C3H6O are polar molecules. Thus, intermolecular interactions will be dipole-dipole interactions.
2.24. Based on solute solvent interactions, arrange the following in order of increasing solubility in n-octane and explain. Cyclohexane, KCl, CH3OH, CH3CN.
Sol: n-octane (C8H18) is a non-polar liquid and solubility is governed by the principle that like dissolve like. Keeping this in view, the increasing order of solubility of different solutes is:
KCl < CH3OH < CH3C=N < C6H12 (cyclohexane).
2.25. Amongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water?
(i) phenol
(ii) toluene
(iii) formic acid
(iv) ethylene glycol
(v) chloroform
(vi) pentanol
Sol: (i) Phenol (having polar – OH group) – Partially soluble.
(ii) Toluene (non-polar) – Insoluble.
(iii) Formic acid (form hydrogen bonds with water molecules) – Highly soluble.
(iv) Ethylene glycol (form hydrogen bonds with water molecules) Highly soluble.
(v) Chloroform (non-polar)- Insoluble.
(vi) Pentanol (having polar -OH) – Partially soluble.
2.26. If the density of lake water is 1·25 g mL-1, and it contains 92 g of Na+ ions per kg of water, calculate the molality of Na+ ions in the lake. (C.B.S.E. Outside Delhi 2008)
Sol: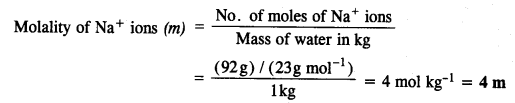
2.27. If the solubility product of CuS is 6 x 10-16, calculate the maximum molarity of CuS in aqueous solution.
Sol: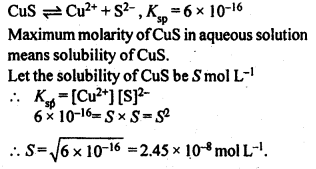
2.28. Calculate the mass percentage of aspirin (C9H8O4 in acetonitrile (CH3CN) when 6.5g of CHO is dissolved in 450 g of CH3CN.
Solution:
2.29. Nalorphene (C19H21NO3), similar to morphine, is used to combat withdrawal symptoms in narcotic users. Dose of nalorphene generally given is 1.5 mg. Calculate the mass of 1.5 x 10-3 m aqueous solution required for the above dose.
Solution: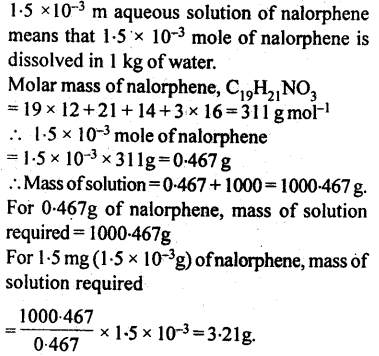
2.30. Calculate the amount of benzoic acid (C5H5COOH) required for preparing 250 mL of 0· 15 M solution in methanol.
Solution: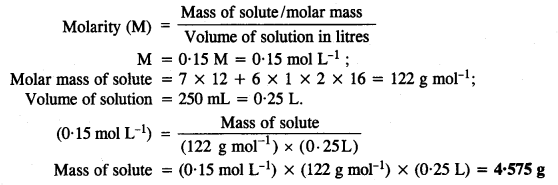
2.31. The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the order given above. Explain briefly.
Solution:
Fluorine being more electronegative than chlorine has the highest electron withdrawing inductive effect. Thus, triflouroacetic acid is the strongest trichloroacetic acid is second most and acetic acid is the weakest acid due to absence of any electron withdrawing group. Thus, F3CCOOH ionizes to the largest extent while CH3COOH ionizes to minimum extent in water. Greater the extent of ionization greater is the depression in freezing point. Hence, the order of depression in freezing point will be CH3COOH < Cl3CCOOH < F3CCOOH.
2.32. Calculate the depression in the freezing point of water when 10g of CH3CH2CHClCOOH is added to 250g of water. Ka = 1.4 x 1o-3 Kg = 1.86 K kg mol-1.
Solution:
2.33. 19.5g of CH2FCOOH is dissolved in 500g of water. The depression in the freezing point of water observed is 1.0°C. Calculate the van’s Hoff factor and dissociation constant of fluoroacetic acid.
Class 12 Chemistry NCERT Solutions Chapter 7 The p Block Elements – Important Questions
Q 1:Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity
Answer
General trends in group 15 elements
(i) Electronic configuration: There are 5 valence electrons for all the elements in group 15.
(ii) Oxidation states: All these elements require three or more electrons to complete their octets and have 5 valence electrons. It is difficult in gaining electrons as the nucleus will have to attract three more electrons. This happens only with nitrogen as it is the smallest in size and the distance between the nucleus and the valence shell is relatively small. The remaining elements of this group show a formal oxidation state of −3 in their covalent compounds. In addition to the −3 state, N and P also show −1 and −2 oxidation states. All the elements present in this group show +3 and +5 oxidation states. However, the stability of +5 oxidation state decreases down a group, whereas the stability of +3 oxidation state increases. This happens because of the inert pair effect.
(iii) Ionization energy and electronegativity
Ionization decreases as we move down the group. This happens because of increase in atomic
sizes. Moving down the group, electronegativity decreases due to increase in size.
(iv) Atomic size: As we move down the group atomic size increases. This increase in
the atomic size is attributed to an increase in the number of shells.
Q 2: Why does the reactivity of nitrogen differ from phosphorus?
Answer
Nitrogen is chemically less reactive. This is because of the high stability of its molecule,
Q 3: Discuss the trends in chemical reactivity of group 15 elements.
Answer
General trends in chemical properties of group − 15
(i) Reactivity towards hydrogen:
The elements of group 15 react with hydrogen to form hydrides of type
(ii)Reactivity towards oxygen:
The elements of group 15 form two types of oxides:
(iii) Reactivity towards halogens:
The group 15 elements react with halogens to form two series of salts:
(iv) Reactivity towards metals:
The group 15 elements react with metals to form binary compounds in which metals exhibit
−3 oxidation states.
Q 4: Why does
When compared to phosphorus nitrogen is highly electronegative. This results in a greater attraction of electrons towards nitrogen in
Q 5: How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions involved
Answer
An aqueous solution of ammonium chloride is treated with sodium nitrite.
NH4Cl (aq ) + NaNO2 →N2(g) + 2H2O(l) + NaCl(aq)
NO and
Q 6: How is ammonia manufactured industrially?
Answer:
Ammonia is prepared on a large-scale by the Haber’s process.
N2(g) + 3H2(g) ⇌ 2NH3(g)

The optimum conditions for manufacturing ammonia are:
(i) Pressure (around
(ii) Temperature (700 K)
(iii) Catalyst such as iron oxide with small amounts of

Q 7: Illustrate how copper metal can give different products on reaction with HNO3.
Answer
Concentrated nitric acid is a strong oxidizing agent. It is used for oxidizing most metals.
The products of oxidation depend on the temperature, concentration of the acid, and also
on the material undergoing oxidation.
3Cu + 8HNO3( dil.) → 3Cu(NO3)2 + 2NO + 4H2O
Cu + 4HNO3( conc. ) →Cu(NO3)2 + 2NO2 +2H2O
Q 8: Give the resonating structures of
Answer
NO2
N2O5
Q 9: The HNH angle value is higher than HPH, HAsH and HSbH angles. Why? [Hint: Can be explained on the basis of
Answer
Hydride
H−M−H angle 107° 92° 91° 90°
The above trend in the H−M−H bond angle can be explained on the basis of the electronegativity of the central atom. Since nitrogen is highly electronegative, there is high electron density around nitrogen. This causes greater repulsion between the electron pairs around nitrogen, resulting in maximum bond angle. We know that electronegativity decreases on moving down a group. Consequently, the repulsive interactions between the electron pairs decrease, thereby decreasing the H−M−H bond angle.
Q 10: Why does
Answer
N (unlike P) lacks the d-orbital. This restricts nitrogen to expand its coordination number
beyond four. Hence,
Q 11: Explain why
Answer
Nitrogen has a small size due to which the lone pair of electrons is concentrated in a small
region. This means that the charge density per unit volume is high. On moving down a
group, the size of the central atom increases and the charge gets distributed over a large
area decreasing the electron density. Hence, the electron-donating capacity of group 15
element hydrides decrease on moving down the group.
Q 12: Nitrogen exists as diatomic molecule and phosphorus as
Answer
Nitrogen owing to its small size has a tendency to form pπ−pπ multiple bonds with itself.
Nitrogen thus forms a very stable diatomic molecule,
Q 13: Write the main differences between the properties of white phosphorus and red phosphorus.
Answer :

Q 14: Why does nitrogen show catenation properties less than phosphorus?
Answer
Catenation is much more common in phosphorous compounds than in nitrogen
compounds. This is because of the relative weakness of the N−N single bond as compared
to the P−P single bond. Since nitrogen atom is smaller, there is greater repulsion of
electron density of two nitrogen atoms, thereby weakening the N−N single bond.
Q 15: Give the disproportionation reaction of
Answer
On heating, orthophosphorus acid (
4H3P+3O3→ 3H3P+5O4 + P-3H3
Q 16: Can
Answer
Q 17: Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in
terms of electronic configuration, oxidation state and hydride formation.
Answer
The elements of group 16 are collectively called chalcogens.
(i) Elements of group 16 have six valence electrons each.
The general electronic configuration of these elements
is
(ii) Oxidation state:
As these elements have six valence electrons (
(iii) Formation of hydrides:
These elements form hydrides of formula
Q 18: Why is dioxygen a gas but sulphur a solid?
Answer
Oxygen is smaller in size when compared to sulphur. Since its size is small, it can form pπ−pπ bonds and form
Q 19: Knowing the electron gain enthalpy values for O → O -1 and O → O 2- as −141 and
(Hint: Consider lattice energy factor in the formation of compounds).
Answer
More the lattice energy of a compound, more stable it will be. Stability of an ionic compound depends on its lattice energy.
Lattice energy is directly proportional to the charge carried by an ion. When a metal combines with oxygen, the lattice energy of the oxide involving O2- ion is much more than the oxide involving O– ion. Hence, the oxide having O2- ions are more stable than oxides having O– ion. Hence, we can say that formation of O2- is energetically more favourable than formation of O–.
Q 20. Which aerosols deplete ozone?
Ans:
The aerosol which is responsible for the depletion of ozone is: Freons or chlorofluorocarbons (CFCs).
The molecules of CFS break down when there is presence of ultraviolet radiations and forms chlorine free radicals which then combines with ozone to form oxygen.
Q 21. Describe the manufacture of
Ans:
The steps which are required in the production of Sulphuric Acid by the contact process
Step (1)
Sulphide ores or Sulphur are burnt in air to form
Step (2)
By a reaction with oxygen,
Step (3)
This oleum is then diluted to obtain
In practice, the plant is operated at 2 bar (pressure) and 720 K (temperature). The sulphuric acid thus obtained is 96-98% pure.
Q 22: How is SO2 an air pollutant?
Soln: The environment is harmed by sulphur dioxide in many ways:
- Sulphuric acid is formed, when it is combined with water vapour present in the atmosphere. This causes acid that damages plants, soil, buildings (those made of marble are more prone), etc.
- SO2 causes irritation in respiratory tract, throat, eyes and can also affect the larynx to cause breathlessness.
- The colour of the leaves of the plant gets faded when it is exposed to sulphur dioxide for a long time. This defect is known as chlorosis. The formation of chlorophyll is affected by the presence of sulphur dioxide.
Q 23: Why are halogens strong oxidising agents?
Soln: Halogens have an electronic configuration of np5, where n =2 to 6. Thus, halogens require only one more electron to complete their octet and to attain the stable noble gas configuration. Moreover, halogens have high negative electron gain enthalpies and are highly electronegative with low dissociation energies. As a result, they have a high tendency to gain an electron. Hence, they act as strong oxidising agents.
Q 24: Explain why fluorine forms only one oxoacid, HOF.
Soln: Fluorine has high electronegativity and small size, hence it forms only one oxoacid i.e HOF.
Q 25: Explain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding while chlorine does not.
Ans : Oxygen has a smaller size and due to which a higher electron density per unit volume. Hence, oxygen forms hydrogen bonds while chlorine does not despite having similar electronegative values.
Q 26. Write two uses of ClO2.
Ans : Applications of ClO2
( a )Used for purification of water.
( b ) Used for bleaching.
Q 27. Why are halogens coloured?
Ans : Halogens are coloured because they take in radiations from the visible spectrum. This excites the valence electrons to a higher energy level. The amount of energy required for excitation differs from halogen to halogen, thus they exhibit different colors.
Q28. Write the reactions of F2 and Cl2 with water.
Ans: ( i ) Cl2 + H2O → HCL + HOCL
( ii ) 2 F2 + 2H2O → 4H+ + 4F- + O2
Q29. How can you prepare Cl2 from HCl and HCl from Cl2? Write reactions only
Ans:
( i ) HCl is prepared from Cl2by reacting it with water.
Cl2 + H2O → HCL + HOCL
( ii ) Cl2 is prepared by Deacon’s process from HCL
4HCL + O2 → 2Cl2 + 2H2O
Q30. What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
Ans: N.Barlett observed that PtF6 and O2 react to produce a compound O2+[ PtF6]–.
As the first ionization enthalpy of Xe( 1170 kJ/mol ) is very close to that of O2 , he figured that PtF6 could also oxidize Xe to Xe+. Thus, he reacted PtF6 and Xe to form a red coloured compound Xe+[ PtF6]– .
Q31. What are the oxidation states of phosphorus in the following:
( a ) H3PO3
( b ) PCl3
( c ) Ca3P2
( d ) Na3PO4
( e ) POF3?
Ans: Let the oxidation state of phosphorous be x
(a) H3PO3
3 + x + 3( -2) = 0
x -3 = 0
x =3
(b) PCl3
x + 3( -1) = 0
x = 3
(c) Ca3P2
3( 2 ) + 2 (x) = 0
2x = -6
x = -3
(d) Na3PO4
3( 1 ) + x + 4( -2 ) = 0
x -5 =0
x =5
(e)POF3
x + ( -2 ) + 3( -1) = 0
x -5 = 0
x = 5
Q 32. Write balanced equations for the following:
(i) NaCl is heated with sulphuric acid in the presence of MnO2.
(ii) Chlorine gas is passed into a solution of NaI in water.
Ans:
(a) 4NaCl + MnO2 + 4H2SO4→ MnCl2 + 4NaHSO4 + 2H2O +Cl2
(b) Cl2 + NaI → 2NaCl + I2
Q33. How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Ans: XeF2, XeF4 and XeF6are obtained through direction reactions between Xe and F2. The product depends upon the conditions of the reaction :
Xe + F2→ XeF2
(excess)
When Xe reacts with F2 under the condition of 673K and 1 bar XeF2 is produced.
Xe + 2F2→ XeF4
( 1:5 ratio )
When Xe reacts with F2 in the ratio of 1:5 under the condition of 873K and 7 bar XeF4 is produced.
Xe + 3F2 → XeF6
(1 : 20 ratio)
When Xe reacts with F2 in the ratio of 1:20 under the condition of 573K and 60-70 bar XeF6 is produced.
Q34. With what neutral molecule is ClO– isoelectronic? Is that molecule a Lewis base?
Ans: ClO– is isoelectronic with ClF.
Total electrons in ClO– = 17 + 8 + 1 =26
Total electrons in ClF = 17 + 9 = 26
As ClF accepts electrons from F to form ClF3 , ClF behaves like a Lewis base.
Q35. How are XeO3 and XeOF4 prepared?
Ans: XeO3 can be obtained using two methods :
( 1 ) 6XeF4 + 12H2O → 4Xe + 2XeO3 + 24HF + 3O2
( 2 ) XeF6 + 3H2O → XeO3 + 6HF
XeOF4 is obtained using XeF6
XeF6 + H2O → XeOF4 + 2HF
Q36. Arrange the following in the order of property indicated for each set:
(i) F2, Cl2, Br2, I2 – increasing bond dissociation enthalpy.
(ii) HF, HCl, HBr, HI – increasing acid strength.
(iii) NH3, PH3, AsH3, SbH3, BiH3 – increasing base strength.
Ans:
(1) Bond dissociation energy normally lowers on moving down a group because of increase in the atomic size. However, F2has a lower bond dissociation energy than Cl2 and Br2. This is because the atomic size of fluorine is very small.
Therefore, the increasing order for bond dissociation enthalpy is:
I2< F2< Br2< Cl2
(2) Bond dissociation energy of a H-X molecule ( where X = F, Cl, Br, I ) lowers with an increase in the size of an atom. As, H-I bond is the weakest it will be the strongest acid.
Therefore, the increasing order acidic strength is :
HF <HCl<HBr< HI
(3) BiH3≤ SbH3<AsH3< PH3< NH3
On moving from nitrogen to bismuth, the atomic size increases but the electron density of the atom decreases. Hence, the basic strength lowers.
Q37. Which one of the following does not exist?
(i) XeOF4 (ii) NeF2 (iii) XeF2 (iv) XeF6
Ans: The one that does not exist is NeF2.
Q38. Give the formula and describe the structure of a noble gas species which is isostructural with:
( a ) ICl4–
( b ) IBr2–
( c ) BrO3–
Ans:
(a) XeF4 is isoelectronic to ICl4– . And it is square planar in geometry :

(b) XeF2 is isoelectronic with IBr2– . It has a linear structure.

(c)XeO3 is isoelectric and isostructural to BrO3–. It has a pyramidal structure.

Q39. Why do noble gases have comparatively large atomic sizes?
Ans:
Noble gases have atomic radii that correspond to van der Waal’s radii. Whereas, other elements have a covalent radius. Now, by definition, van der Waal’s radii are bigger than covalent radii. This is the reason why noble gases have relatively bigger atomic sizes.
Q40. List the uses of neon and argon gases.
Ans: Uses of Argon gas:
(a) Argon is used to keep an inert atmosphere in high temperature metallurgical operations like arc welding.
(b) It is used in fluorescent and incandescent lamps where it is required to check the sublimation of the filament. Thereby, increasing the life of the lamp.
(c) Argon is used in laboratories to handle substances that are air-sensitive.
Uses of neon gas:
(a) Neon is filled in discharge tubes for advertising or decoration.
(b) Neon is used for making beacon lights.
(c) It is used alongside helium to protect electrical equipment against high voltage.
The NCERT Solutions for Class 12 Chemistry Chapter 7 deals with the chemistry of inorganic ring systems of the p-block elements has a long and venerable history that dates back to the early 19th century. The elements of Group 13, 14, 15, 16, 17 and 18 are called P block elements. They are represented by the general outer electronic configuration ns2np1-6. P block elements exist in all three physical states and maybe metals, non-metals or metalloids. Access the NCERT Solutions of this chapter to learn more in detail.
Class 12 Chemistry NCERT Solutions for Chapter 7 The p Block Elements
Chapter 7 p-Block Elements of Class 12 Chemistry is categorized under the term – I CBSE Syllabus for the session 2021-22. The NCERT Solutions for Class 12 are provided here for better understanding and clarification of the concepts. It is the best reference material when it comes to learning and understanding complex concepts/problems.
Subtopics of Class 12 Chemistry Chapter 7 – The P Block Elements
- Group 15 Elements
- Dinitrogen
- Ammonia
- Oxides of Nitrogen
- Nitric Acid
- Phosphorus — Allotropic Forms
- Phosphine
- Phosphorus Halides
- Oxoacids of Phosphorus
- Group 16 Elements
- Dioxygen
- Simple Oxides
- Ozone
- Sulphur — Allotropic Forms
- Sulphur Dioxide
- Oxoacids of Sulphur
- Sulphuric Acid
- Group 17 Elements
- Chlorine
- Hydrogen Chloride
- Oxoacids of Halogens
- Interhalogen Compounds
- Group 18 Elements
Nitrogen comprises 78% by volume of the atmosphere. Oxygen which is the most abundant element on earth forms 46.6% of the mass of the earth’s crust. Fluorine is present in insoluble fluorides. Seawater contains bromides, chlorides and iodides of potassium, calcium, magnesium and sodium. It is mainly sodium chloride solution. Learn more by accessing the NCERT Solutions for Class 12 Chemistry for free.
Frequently Asked Questions on NCERT Solutions for Class 12 Chemistry Chapter 7
What type of questions can I answer by referring to the NCERT Solutions for Class 12 Chemistry Chapter 7?
What are Group 15 Elements?
Group 15 elements are also called Nitrogen family includes nitrogen
phosphorus, arsenic, antimony and bismuth elements. The p-block elements
are also known as the Representative
Elements which is placed on the right side of the main
periodic table.
The modern periodic table as conceived by Dimitri Mendeleev arranges
all the elements known to man on the basis of its atomic number, which is
unique to every element. The results of such an arrangement were the periodic
table. The elements with similar properties were arranged into a
column called a group.
Periodic Trends in Group 15 Elements
So in Group 15 elements as you would move down a group, starting
with the lightest element and finishing with the heavy ones; you’d notice a
general flow in properties as you move down the order. For eg, Nitrogen is a
gas and non-metal but as you move down the group, we encounter metalloids and
then at the bottom, metal i.e. Bismuth. These trends in the periodic table help
us better understand the behaviour of atoms and also helps us predict new
elements.
|
Property |
Nitrogen |
Phosphorus |
Arsenic |
Antimony |
Bismuth |
|
Atomic symbol |
N |
P |
As |
Sb |
Bi |
|
Atomic number |
7 |
15 |
33 |
51 |
83 |
|
Atomic mass (amu) |
14.01 |
30.97 |
74.92 |
121.76 |
209.98 |
|
Valence electron configuration |
[He]2s2 2p3 |
[Ne]3s2 3p3 |
[Ar]3d10 4s24p3 |
[Kr]4d10 5s25p3 |
[Xe]4f14 5d106s26p3 |
|
Melting point Boiling
point (°C) |
– 210 -196 |
44.15 281 |
817 603(sublimes) |
631 1587 |
271 1564 |
|
Density (g/cm3) at 25°C |
1.15(g/L) |
1.8 |
5.7 |
6.68 |
9.79 |
|
Atomic radius (pm) |
56 |
98 |
114 |
133 |
143 |
|
First Ionization energy (kJ/mol) |
1402 |
1012 |
947 |
834 |
703 |
|
Oxidation state(s) |
-3 to +5 |
+5, +3, -3 |
+5, +3 |
+5, +3 |
+3 |
|
Ionic radius (pm) |
146(-3) |
212(-3) |
58(+3) |
76(+3) |
103(+3) |
|
Electronegativity |
3.0 |
2.2 |
2.2 |
2.1 |
1.9 |
Some of the trends in the modern periodic table with respect to
group 15 elements of the p-Block elements are discussed below.
1. Electronic Configuration
·
The valence shell
electronic configuration plays a major role in how an element behaves.
The valence electron shell configuration of
group 15 elements is ns2np3.
·
All the group 15
elements have the same arrangement and this is why they’re similar.
·
The s-orbital in this
group is completely filled and the
p-orbitals are half filled and this makes their configuration extra stable.
2. Atomic and Ionic Radii
·
If you see the
electronic configuration of elements in the table above, you will notice that
with every step you move downwards, new orbitals are added to the atom.
·
This addition of new
orbitals increases both the Atomic and the Ionic radii of group 15 elements.
·
However, we see that
from Arsenic to Bismuth only a small increase in ionic radius is observed.
·
This is due to the
presence of completely filled d and/or f orbitals in heavier members.
3. Ionization Enthalpy
·
Ionization Energy is the
amount of energy required to remove an electron from the outermost orbit of the
atom.
·
This is basically a
measure of how hard the nucleus is holding on to the electron.
·
The closer the electron
is to the nucleus the stronger its hold and thus the energy required is more.
·
As we move down the
group, the radius of the atom increases and therefore the Ionization
energy decreases due to the weaker hold of the nucleus.
4. Electronegativity
·
The electronegativity
value decreases down the group with increasing atomic size.
·
This again is due to the
increasing distance between the nucleus and the valence shell as we move down
the group.
5. Physical Properties
·
All the elements of the
group exist in a polyatomic state.
·
The first, Nitrogen is
gas but as you move down there is a significant increase in the metallic
character of the elements.
·
Nitrogen and Phosphorus
are non-metals, Arsenic and Antimony are metalloids and Bismuth is a metal.
·
These changes can be
attributed to the decrease in Ionization enthalpy and increase in atomic size.
Uses of Argon
Argon is used mainly to provide an inert atmosphere in high temperature metallurgical processes such as arc welding of metals and alloys. In the laboratory, it is used for handling substance which are air sensitive.It is used in filling incandescent and fluorescent lamps where its presence retards the sublimation of the filament and thus increases the life of the lamp.It is also used in “neon signs” for obtaining lights of
DR
OMAR CLASSES BARRA KANPUR MOB 9450149685
DR
OMAR CLASSES BARRA KANPUR MOB 9450149685
·
Boiling points also, in
general, show an increasing trend as you move down.
·
Except for Nitrogen, all
the other elements have allotropes.
6. Chemical Properties
·
The valence shells of
the p-Block elements have a configuration of
ns2 np3.
·
So the elements here can
either lose 5 electrons or gain 3.
·
The common oxidation
states of these elements are -3, +3 and +5.
·
With a decrease in the
Ionization enthalpy and electronegativity, due to the increasing atomic radius,
the tendency to gain three electrons to create a -3 oxidation state decreases
down the group.
·
In fact, Bismuth hardly
forms any compounds with -3 oxidation state.
·
As we go down, the
stability of the +5 state decreases and that of +3 increases due to inert pair
effect.
Apatite Family
Apatite families can be described as a group of similar
isomorphous hexagonal phosphate minerals. The main apatite group consists of
Fluorapatite, Chlorapatite, and Hydroxylapatite. The teeth and bones of various
animals, including humans, are composed of Calcium phosphate, which is also the
same material as apatite.
The primary Apatite group includes Fluorapatite, Chlorapatite,
and Hydroxylapatite. The extended Apatite supergroup describes additional
minerals such as Pyromorphite, Mimetite, and Vanadinite. Apatite is the main
source of phosphorous, an important nutrient required by plants. As such,
apatite is the key ingredient in phosphate fertilizers. Most of the phosphorus
used in fertilizer comes from phosphate rock, which is mined almost exclusively
for this application.
Why is Group 15
called P block?
It is a p-block element since it takes the physical and chemical
properties after that of other p-block elements of the eighteenth group.
P-block elements are generally non-metals, while the remaining are metalloids
and metals.
What is the other
name of group 15 elements?
This group is also known as the nitrogen family. It consists of
the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth
(Bi), and perhaps the chemically uncharacterized synthetic element moscovium
(Mc). In modern IUPAC notation, it is called Group 15.
Why do all Group
15 elements have similar chemical properties?
Each element within a group has similar physical or chemical
properties because of its atom’s outermost electron shell (most chemical
properties are dominated by the orbital location of the outermost electron).
What element
family does oxygen belong to?
Chalcogens. Group 6A (or VIA) of the periodic table are the
chalcogens: the nonmetals oxygen (O), sulfur (S), and selenium (Se), the
metalloid tellurium (Te), and the metal polonium (Po).
What do we use
nitrogen for?
Nitrogen is important to the chemical industry. It is used to
make fertilizers, nitric acid, nylon, dyes and explosives. To make these
products, nitrogen must first be reacted with hydrogen to produce ammonia.
Maths and Chemistry By – Dr.V.K.Omar
DR OMAR CLASSES BARRA KANPUR
MOB 9450149685
What are the benefits of using the NCERT Solutions for Class 12 Chemistry Chapter 7
1. The solutions created are completely based on the latest term – I CBSE Syllabus and its guidelines.
2. The subject matter experts use simple and understandable language to help students grasp the concepts effectively.
3. Students obtain a strong foundation of fundamental concepts which are important from the first term exam point of view.
4. By regular practice, students will be able to answer complex questions without any difficulty.
What are the important topics covered in Chapter 7 of NCERT Solutions for Class 12 Chemistry?
1. Group 15 Elements
2. Dinitrogen
3. Ammonia
4. Oxides of Nitrogen
5. Nitric Acid
6. Phosphorus — Allotropic Forms
7. Phosphine
8. Phosphorus Halides
9. Oxoacids of Phosphorus
10. Group 16 Elements
11. Dioxygen
12. Simple Oxides
13. Ozone
14. Sulphur — Allotropic Forms
15. Sulphur Dioxide
16. Oxoacids of Sulphur
17. Sulphuric Acid
18. Group 17 Elements
19. Chlorine
20. Hydrogen Chloride
21. Oxoacids of Halogens
22. Interhalogen Compounds
23. Group 18 Elements
NCERT IN TEXT QUESTIONS
7.1. Why are pentahalides more covalent than trihalidcs?
Ans: The group 15 elements have 5 e-1 s in their valence shell. It is difficult to lose 3e-1s to form E3+ and even more difficult to lose 5e-1 s to form E5+. Thus, they have very little tendency to form ionic compounds. Further, since the elements in +5 state have less tendency to lose e-1s than in the +3 state, elements in +5 state have more tendency to share e-1 s and hence pentahalides are more covalent than trihalides.
7.2. Why is BiH3 the strongest reducing agent amongst all the hydrides of group 15 elements? (C.B.S.E. 2013)
Ans: Down the group, the atomic size of the element (E) increases and the bond length of the corresponding E—H bond also increases. This adversely affects the bond dissociation enthalpy. This means that amongst the trihydrides of the members of nitrogen family, the bond dissociation enthalpy of Bi—H bond is the least. Therefore, BiH3 is the strongest reducing agent among the hydrides of group 15 elements.
7.3. Why is N2 less reactive at room temperature?
Ans: Due to presence of triple bond between two N-atoms (N = N), the bond dissociation energy of N2 is very high. As a result, N2 becomes less reactive at room temperature.
7.4. Mention the conditions required to maximise the yield of ammonia.
Ans: Ammonia is prepared by Haber’s process as given below: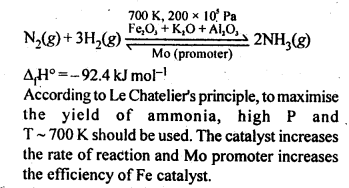
7.5. How does ammonia react with a solution of Cu2+?
Ans: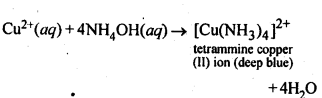
7.6. What is the covalence of nitrogen in N2O5 ?
Ans: In N2O5 , each N-atom has four shared pairs of e-1 s as shown: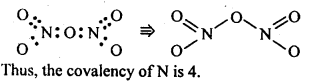
7.7. Why is bond angle in
Ans: In both PH3 and 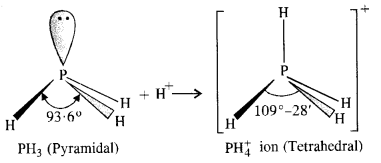
Because of lone pair : shared pair repulsion which is more than that of shared pair : shared pair repulsion, the bond angle in PH3 is nearly 93-6°. In
7.8. What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2?
Ans: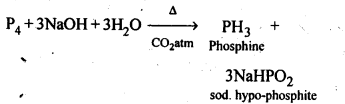
7.9. What happens when PCl5 is heated?
Ans:
7.10. Write a balanced equation for the hydrolytic reaction of PC is in heavy water.
Ans: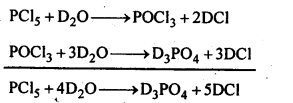
7.11. What is the basicity of H3PO4?
Ans: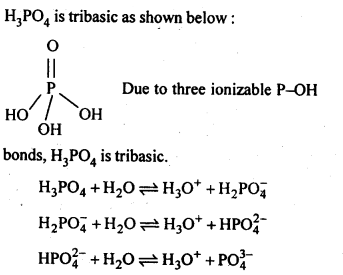
7.12. What happens when H3PO4 is heated?
Ans: On heating, H3PO3 disproportionates to form PH3 and H3PO4 with O.S. of-3and + 5.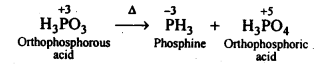
7.13. List the important sources of sulphur.
Ans: Sulphur mainly occurs in the combined states in earth’s crust in the form of sulphates and sulphides.
Sulphates : gypsum (CaSO4.2H2O); epsom (MgSO4.7H2O); baryte (BaSO4), etc.
Sulphides : Galena (PbS); zinc blende (ZnS); copper pyrites (CuFeS2); iron pyrites (FeS2), etc. Traces of sulphur occur’as H2S and in organic materials such as eggs, proteins, garlic, onion, mustard, hair and wool.
7.14. Write the order of thermal stability of the – hydrides of Group 16 elements.
Ans: The thermal stability of hydrides of group 16 elements decreases down the group. This is because down the group, size of the element (M) increases, M-H bond length increases and thus, stability of M-H bond decreases so that it can be broken down easily. Hence, we have order of thermal stability as H2O > H2S > H2Se > H2Te > H2PQ
7.15. Why is H2O a liquid and H2S a gas?
Ans: Due to high electronegativity of O than S, H2O undergoes extensive intermolecular H-bonding. As a result, H2O exists as an associated molecule in which each O is tetrahedrally surrounded by four H2O molecules. Therefore, H2O is a liquid at room temperature.
On the other hand,H2S does not undergo H- bonding. It exists as discrete molecules which are held together by weak van der waals forces of attraction. A small amount of energy is required to break these forces of attraction. Therefore, H2S is a gas at room temperature.
7.16. Which of the following does not react with oxygen directly? Zn, Ti, Pt, Fe
Ans: Platinum (Pt) is a noble metal and does not react with oxygen directly.
7.17. Complete the following reactions:
(i)C2H2 + O2 -> (ii) 4Al + 3 O2 ->
Ans:
7.18. Why does O3 act as a powerful oxidising agent?
Ans: On heating, O3 readily decomposes to give O2 and nascent oxygen.![]()
Since nascent oxygen is very reactive, therefore, O3 acts as a powerful oxidising agent.
7.19. How is O3 estimated quantitatively?
Ans: When O3 is treated with excess of KI solution buffered with borate buffer (pH = 9.2), I2 is liberated quantitatively.![]()
The I2 thus liberated is titrated against a standard solution of sodium thiosulphate using starch as an indicator.![]()
7.20. What happens when sulp’hur dioxide is passed through an aqueous solution of Fe(III) salt?
Ans: SO2 acts as a reducing agent and reduces aqueous solution of Fe (III)salt to Fe (II) salt.
7.21. Comment on the nature of two S-O bonds formed in S02 molecule. Are the two S-O bonds in this molecule equal ?
Ans: SO2 exists as an angular molecule with OSO bond angle of 119.5°. It a resonance hybrid of two canonical-forms: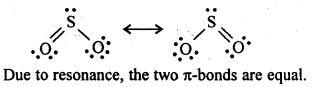
7.22. How is the presence of SO2 detected?
Ans: SO2 is a pungent smelling gas. It can be detected by two test:
7.23. Mention three areas in which H2SO4 plays an important role.
Ans: (i) Sulphuric acid is used for the manufacture of a number of chemicals like hydrochloric acid, phosphoric acid, nitric acid along with a large number of organic compounds.
(ii) A mixture of concentrated nitric acid and concentrated sulphuric acid is used in the manufacture of explosives like picric acid, T.N.T, dynamite etc.
(iii) Dilute solution of acid is employed in petroleum refining in order to remove the unwanted impurities of sulphur.
Question 24.
Write the conditions to maximise the yield of H2SO4 by Contact process.
Solution:
The key step in the manufacture of sulphuric acid is oxidation of SO2 to SO3 in presence of V2O5 catalyst.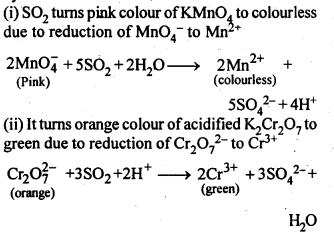
The reaction is exothermic and reversible. Hence, low temperature and high pressure are the favourable conditions for maximum yield of SO3. In practice a pressure of 2 bar and temperature of 720 K is maintained.
Question 25.
Why is Ka2 « Ka1 for H2SO4 in water?
Solution:
H2SO4 is a very strong acid in water largely because of its first ionisation to H3O+ and HSO4– The ionisation of HSO4– to H3O+ and SO42- is very very small. That is why, Ka2« Ka1.
Question 26.
Considering the parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy, compare the oxidising powers of F2 and Cl2.
Solution:
The oxidising powers of both the members of halogen family are expressed in terms of their electron accepting tendency and can be compared as their standard reduction potential values.
F2 + 2e– → 2F–; E° = 2-87 V, Cl2 + 2e– → 2Cl– ; E° = 1-36 V
Since the E° of fluorine is more than that of chlorine, it is a stronger oxidising agent.
Explanation : Three factors contribute towards the oxidation potentials of both the halogens. These are :
(i) Bond dissociation enthalpy: Bond dissociation enthalpy of F2 (158 kJ mol-1) is less compared to that of Cl2 (242·6 kJ mol-1).
(ii) Electron gain enthalpy: The negative electron gain enthalpy of F (- 332·6 kJ mol-1) is slightly less than of Cl (-348·5 kJ mol-1).
(iii) Hydration enthalpy: The hydration enthalpy of F- ion (515 kJ mol-1) is much higher than that of Cl- ion (381 kJ mol-1) due to its smaller size.
From the available data, we may conclude that lesser bond dissociation enthalpy and higher hydration enthalpy compensate lower negative electron gain enthalpy of fluorine as compared to chlorine. Consequently, F2 is a more powerful oxidising agent than Cl2.
Question 27.
Give two examples to show the anomalous behaviour of fluorine.
Solution:
- Ionisation enthalpy, electro-negativity and electrode potential are higher for fluorine than the expected trends of other halogen.
- Fluorine does not show any positive oxidation state except in HOF.
Question 28.
Sea is the greatest source of some halogens. Comment.
Solution:
Sea water contains chlorides, bromides and iodides of sodium, potassium, magnesium and calcium but sodium chloride being the maximum makes sea water saline. Various sea weeds contain upto 0.5% iodine.
Question 29.
Give the reason for bleaching action of Cl2.
Solution:
Chlorine bleaches by oxidation Cl2 + H2O → HCl + HOCl → HCl + [O]
The nascent oxygen reacts with dye to make it colourless.
Question 30.
Name two poisonous gases which can be prepared from chlorine gas.
Solution:
COCl2 (phosgene), CCl3NO2 (tear gas)
Question 31.
Why is ICI more reactive than l2?
Solution:
In general, interhalogen compounds are more reactive than halogens due to weaker X-X’ bonding than X-X bond. Thus, ICI is more reactive than I2.
Question 32.
Why is helium used in diving apparatus?
Answer:
Helium along with oxygen is used in the diving apparatus by the sea divers. Since it is very little soluble in blood, it reduces decompression and causes less discomfort to the diver in breathing. A mixture of helium and oxygen does not cause pain due to very low solubility of helium in blood as compared to nitrogen.
Question 33.
Balance the following equation :
XeF6 + H2O → XeO2F2 + 4HF
Solution:
Question 34.
Why has it been difficult to study the chemistry of radon?
Solution:
Radon is radioactive with very short half-life which makes the study of chemistry of radon difficult.
NCERT EXERCISES
7.1. Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Sol: In group 15 of the Periodic Table, the elements, nitrogen (7N), phosphorus (15P), arsenic (33As), antimony (51Sb) and bismuth (83Bi) are present. The elements of this group can exhibit various oxidation states ranging between -3 to + 5. Negative oxidation state will be exhibited when they combine with less electronegative element andpositive oxidation state will be exhibited with more electronegative element. Positive oxidation state becomes more favourable as we more down the group due to increasing metallic character & electropositivity. Although due to inert pair effect the stability of +5 state will also decrease. The only stable compound of Bi (V) is BiF5.
The atomic (covalent) and ionic radii (in a particular oxidation state) of the elements of nitrogen family (group 15) are smaller than the corresponding elements of carbon family (group 14). On moving down the group, the covalent and ionic radii (in a particular oxidation state) increase with increase in atomic number. There is a considerable increase in covalent radius from N to P. However, from As to Bi, only a small increase is observed.
As the size increases on moving down the group, the ionisation enthalpy increases. The ionisation enthalpy of nitrogen group elements is more than the corresponding elements of oxygen group. This is because of more stable half-filled outermost p- subshell of nitrogen group elements. Electronegativity decreases down the group with increase in atomic size.
7.2. Why is the reactivity of nitrogen different from that of phosphorus?
Sol: Molecular nitrogen exists as a diatomic molecule (N2) in which the two nitrogen atoms are linked to each other by triple bond (N≡N). It is a gas at room temperature. Multiple bonding is not possible in case of phosphorus due to its large size. It exists as P4 molecule (solid) in which P atoms are linked to one another by single covalent bonds. Because of greater bond dissociation enthalpy (946 kJ mol-1) of N≡N bond, molecular nitrogen is very less reactive as compared to molecular phosphorus.
7.3. Discuss the trends in chemical reactivity of group 15 elements.
Sol: Hydrides: All elements of group 15 form gaseous hydrides of the type MH3.
In all the hydrides the central atom is sp3 hybridized and their shape is pyramidal due to presence of lone pair of electrons.
(a)The basic strength of the hydrides decreases as we move down the group.
Thus, NH3 is the strongest base.
NH3 > PH3 > AsH3 > SbH3
(b)The thermal stability of the hydrides decreases as the atomic size increases, i.e., the M – H bond strength decreases which means reducing character increases.
(c)In the liquid state, the molecules of NH3are associated due to hydrogen bonding. The molecules of other hydrides are not associated.
(d)NH3 is soluble in water whereas other hydrides are insoluble.
(e)All the hydrides, except NH3, are strong reducing agents and react with metal ions (Ag+, Cu2+, etc.) to form phosphides, arsenides or antimonides.
Halides: The elements of group 15 form two series of halides MX3 and MX5.
(a)All the elements of the group form trihalides. The ionic character of trihalides increases as we move down the group. Except NCl3 all the trihalides are hydrolysed by water. This is due to the absence of d-orbitals in nitrogen.
(b)PF3 is not hydrolysed because fluorine being more electronegative than oxygen forms more stable bonds with phosphorus than P – O bonds.
(c)N cannot form NX5 because of non-availability of rforbitals. Bi cannot form BiX3 because of reluctance of 6s electrons of Bi to participate in bond formation.
(d)The hybridisation of M in MX3 is sp3 and shape is pyramidal. M in MX5 is sp3 as hybridised and shape is trigonal pyramidal. The axial bonds in MX5 are weaker and longer, So MX5 are less stable and decompose on heating eg:![]()
Oxides:
(a)Nitrogen forms a number of oxides. The rest of the members (P, As, Sb and Bi) of the group form two types of oxides : E203 and E2O5.
(b)The reluctance of P, As, Sb and Bi to enter into pπ -pπ multiple bonding leads to cage structures of their oxides and they exist as dimers, E4O6 and E5O10.
(c)The basic nature of die oxides increases with increase in atomic number of the element. Thus, the oxides of nitrogen (except N20 and NO), P (III) and As (III) are acidic, Sb (III) oxide is amphoteric and Bi (III) oxide is basic.
7.4. Why does NH3 form hydrogen bond but PH3 does not?
Sol: Nitrogen has an electronegativity value 3.0, which is much higher than that of H (2.1). As a result, N – H bond is quite polar and hence NH3 undergoes intermolecular H – bonding.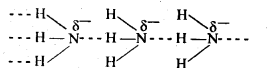
Phosphorus have an electronegativity value 2-1. Thus, P – H bond is not polar and hence PH3 does not undergo H – bonding.
7.5. How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions . involved.
Sol: In laboratory, nitrogen is prepared by heating an equimolar aqueous solution of ammonium chloride and sodium nitrite. As a result of double decomposition reaction, ammonium nitrite is formed. Ammonium nitrite is unstable and decompose to form nitrogen gas.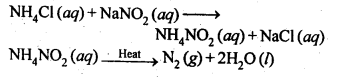
7.6. How is ammonia manufactured industrially?
Sol: Commercially, by Haber’s process.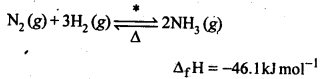
iron oxide, K2O, Al203 The optimum conditions for the production of NH3 are pressure of 200 atm and temperature of 100K.
7.7. Illustrate how copper metal can give different products on reaction with HN03.
Sol: On heating with dil HN03, copper gives copper nitrate and nitric oxide.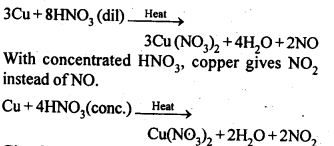
7.8. Give the resonating structures of N02 and N2O5.
Sol: Resonating structures of N02 are: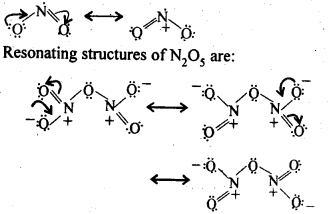
7.9. The HNH angle value is higher than HPH, H AsH and HSbH angles. Why?
(Hint: Can be explained on the basis of sp3 hybridisation in NH3 and only s-p bonding , between hydrogen and other elements of the group).
Sol: In all these cases, the central atom is sp3 hybridized. Three of the four sp3 orbitals form three σ-bonds, while the fourth contains the lone pair of electrons. On moving down from N to Sb, the electronegativity of the central atom goes on decreasing. As a result of this, bond pairs of electrons lie away and away from the central atom. This is because of the force of repulsion between the adjacent bond pairs goes on decreasing and the bond angles keep on decreasing from NH3 to SbH3. Thus, bond angles are in the order:![]()
7.10. Why does R3P=0 exist but R3N=0 does not (R is an alkyl group) ?
Sol: Nitrogen does not have vacant d-orbitals on its valence shell. Therefore, it cannot extend its dπ-pπ bonding is not possible. As a result, the molecules of R3N = 0 does not exist. However, phosphorus and rest of the members of the group 15 have vacant d-orbitals in the valence shell which can be involved in dπ-pπ bonding. Under the circumstances, R3P=0 molecule can exist.
7.11. Explain why NH3 is basic while BiH3 is only feebly basic.
Sol: In both NH3 and BiH3, N and Bi have a lone paif of electrons on the central atom and hence should behave as Lewis bases. But NH3 is much more basic than BiH3. Since the atomic size of N is much smaller than that of Bi, therefore, electron density on N-atom is much higher than that on Bi-atom. Thus, the tendency of N in NH3 to donate its lone pair of electrons is much more in comparison to tendency of Bi in BiH3. Hence, NH3 is more basic than BiH3.
7.12. Nitrogen exists as diatomic molecule and phosphorus as P4. Why?
Sol: Nitrogen exists as a diatomic molecule having a triple bond between the two N-atoms, This is due its small size that it forms pπ-pπ multiple bonds with itself and with carbon /oxygen as well. On the other hand, phosphorus due to its larger size does not form multiple pπ-pπ bonds with itself. It prefers to form P – P single bonds and hence it exists as tetrahedral P4 molecule.
7.13. Write main differences between the properties of white phosphorus and red phosphorus.
Sol: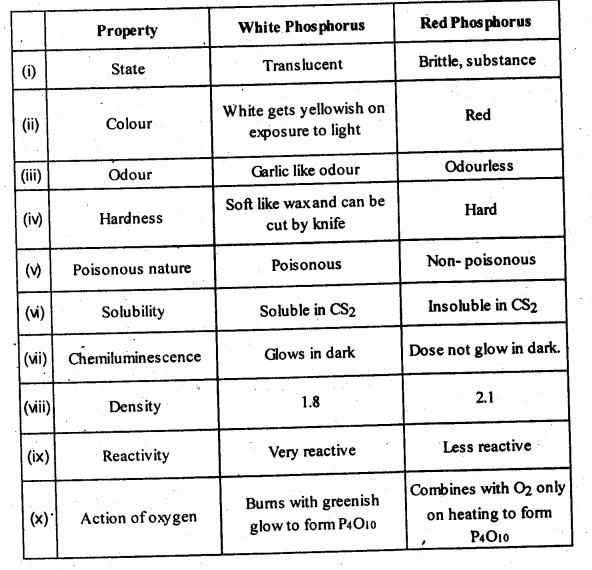
Structure of white and red phosphorus are given below: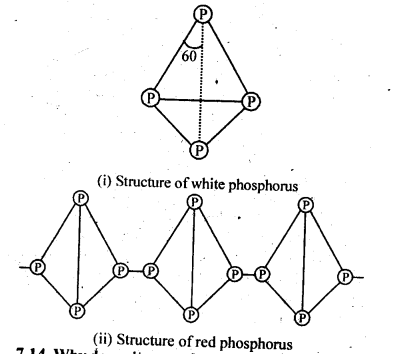
7.14. Why does nitrogen show catenation properties less than phosphorus ? (C.B.S.E. Foreign 2009)
Sol: The valence shell electronic configuration of N is 2s22p3. In order to complete the octet, the two nitrogen atoms share three electron pairs in the valence p-sub-shell and get linked by triple bond (N=N). Thus molecular nitrogen exists as discrete diatomic species and there is no scope of any self linking or catenation involving a number of nitrogen atoms. However, in case of phosphorus, multiple bonding is not feasible due to comparatively large atomic size of the element. Molecular phosphorus exists as tetra-atomic molecule (P4) in white phosphorus. These tetrahedrons are further linked by covalent bonds to form red variety which is in polymeric form. Thus, catenation in nitrogen is less than in phosphorus.
7.15. Give the disproportionation reaction of H3 P03.
Sol: On heating, H3 P04 undergoes self – oxidation-reduction, i.e: disproportionation to form PH3.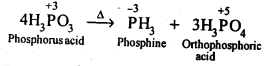
7.16. Can PCl5 act as an oxidising as well as a reducing agent Justify.
Sol: The oxidation state of P in PCl5 is+5. Since P has five electrons in its valence shell, therefore, it cannot donate electron and cannot increase its oxidation state beyond + 5, Thus, PCl5 cannot act as a reducing agent. It can act as oxidizing agent by itself undergoing reduction.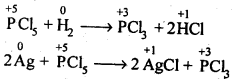
7.17. Justify the placement of O, S, Se, Te and Po in the same group’of the periodic table in terms of electronic configuration, oxidation state and hydride formation.
Sol: (1)Electronic configuration:
O (At. no. = 8) = [He] 2s2 2p4
S (At. no. = 16) = [Ne] 3s2 3p4
Se (At. no. = 34) = [Ar] 3d10 4s2 4p4
Te (At. no. = 52) = [Kr] 4d10 5s2 5p4 ,
Po (At. no. = 84) = [Xe] 4f14 5d10 6s2 6p4 ,
Thus, all these elements have the same ns2 np4 (n = 2 to 6) valence shell electronic configuration, hence are justified to be placed in group 16 of the Periodic Table.
(2)Oxidation state : Two more electrons are needed to acquire the nearest noble gas configuration. Thus, the minimum oxidation state of these elements should be – 2. O and to some extent S show – 2 oxidation state. Other element being more electropositive than O and S, do not show negative oxidation state. As these contain six electrons, thus, maximum oxidation state shown by them is+ 6. Other oxidation state shown by them are + 2 and + 4. O do not show+4 and + 6 oxidation state, due to the absence of d-orbitals. Thus, on the basis of maximum and minimum oxidation states, these elements are justified to be placed in the same group 16 of the periodic table.
(3)Hydride formation: All these elements share two of their valence electrons with 1 s- orbital of hydrogen to form hydrides of the general formula EH2, i.e., H20, H2S, H2Se, H2Te and H2Po. Thus, on the basis of hydride formation, these elements are justified to be placed in the same group 16 of the Periodic Table.
7.18. Why is dioxygen a gas but sulphur a solid?
Sol: Due to the small size and high electronegativity, oxygen forms pπ- pπ multiple bonds. As a result, oxygen exists as diatomic (O2) molecules. These molecules are held together by weak van der Waal’s forces of attraction which can be overcome by collisions of the molecules at room temperature. Therefore, O2 is a gas at room temperature. Due to its bigger size and lower electronegativity, sulphur does not form pn-pn multiple bonds. It prefers to form S – S single bonds. S – S single bond is stronger then O-O single bond. Thus, sulphur has higher tendency for catenation than oxygen. Due to higher tendency for catenation and lower tendency for pπ – pπ multiple bonds sulphur exits as octa-atomic (Sg) molecule. Due to bigger size, the force of attraction holding the Sg molecules together are much stronger which cannot be overcome by collisions of molecules at room temperature. Therefore, sulphur is solid at room temperature.
7.19. Knowing the electron gain enthalpy values of O—>O– and O—>O2- as -141 and 702 kJ mol-1 respectively, how can you account for the formation of a large number of oxides having O2- species and not O–?
Sol: Let us consider the reaction of oxygen with monopositve metal, we can have two compounds. MO(O in -1 state) and M2O (O in -2 state). The energy required for formation of O-2 is compensated by increased coulombic attraction between M+ and O-2. Coulombic force of attraction, FA is proportional to product of charges on ions i.e.
where q1 and q2 are charges on ions and r is distance between ions. Same logic can be applied if metal is dispositive.
7.20. Which aerosols deplete ozone?
Sol: Aerosols like chlorofluorocarbons (CFC’s), i.e., freon (CCl2F2), depletes the ozone layer by supplying Cl* free radicals which convert O3 to O2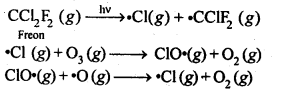
7.21. Describe the manufacture of H2SO4 by contact process?
Sol: Preparation of sulphuric acid:By Contact Process: Burning of sulphur or sulphide ores in presence of oxygen to produce SO2. Catalytic oxidation of SO2 with O2to give SO3 in the presence of V2O5.![]()
Then SO3 made to react with sulphuric acid of suitable normality to obtain a thick oily liquid called oleum.![]()
Then oleum is diluted to obtain sulphuric acid of desired concentration.![]()
The sulphuric acid obtained by contact process is 96-98% pure.
7.22. How is SO2 an air pollutant?
Sol: (1) SO2 dissolves in moisture present in air to form H2SO4 which damages building materials especially marble (acid – rain).- CaCO3 + H2SO3 ——->CaSO3 + H20 + CO2
(2)It corrodes metals like Fe and steel. It also brings about fading and deterioration of fabrics, leather, paper, etc., and affecting the colour of paints.
(3)Even in low concentration (= 0.03 ppm), it has damaging effect on the plants. If exposed for a long time, i.e., a few days or weeks, it slows down the formation of chlorophyll i. e., loss of green colour. This is called chlorosis.
(4)It is strongly irritating to the respiratory track. It cause throat and eye irritation, resulting into cough, tears and redness in eyes. It also cause breathlessness and effects larynx i. e. „ voice box.
7.23. Why are halogens strong oxidising agents?
Sol: Members of the halogen family act as strong oxidising agents on account of their electron accepting tendency both in the molecular as well as atomic form.![]()
This is attributed to their high electronegativity, negative electron gain enthalpy values and alsp low bond dissociation enthalpies sinve they contain single covalent bonds(X — X) in their molecules. Fluorine is most reactive among the halogens and the reactivity down the group.
7.24. Explain why fluorine forms only one oxoacid, HOF.
Sol: Cl, Br and I form four series of oxo acids of general formula HOX, HOXO, HOXO2, and H0XO3. In these oxo-adds, the oxidation states of halogens are + 1, + 3, + 5, and + 7 respectively. However, due to high electronegativity, small size and absence of d-orbitals, F does not form oxo-acids with + 3, + 5 and + 7, oxidation states. It just forms one oxo-acid (HOF).
7.25. Explain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding while chlorine does not.
Sol: Both .nitrogen (N) and chlorine (Cl) have electronegativity of 3.0. However, only nitrogen is involved in the hydrogen bonds (e.g., NH3) and not chlorine. This is due to smaller atomic size of nitrogen (atomic radius =70 pm) as compared to chlorine (atomic radius = 99) pm), therefore, N can cause greater polarisation of N-H bond than Cl in case of Cl—H bond.Consequently, N atom is involved in hydrogen bonding and not chlorine.
7.26. Write two uses of ClO2
Sol: (1) ClO2 is an excellent bleaching agent. It is 30 times stronger bleaching agent then the Cl2. It is used as a bleaching agerit for paper pulp in paper industry and in textile industry. (2) ClO2 is also a powerful oxidising agent and chlorinating agent. It acts as a germicide for disinfecting water. It is used for purifying drinking water.
7.27. Why are halogens coloured?
Sol: The halogens are coloured because their molecules absorb light in the visible region. As a result of which their electrons get excited to higher energy levels while the remaining light is transmitted. The color of halogens is the color of this transmitted light.
7.28. Write the reactions of F2 and Cl2 with water.
Sol:
7.29. How can-you prepare Cl2 from HCl and HCl from CI2? Write reactions only.
Sol: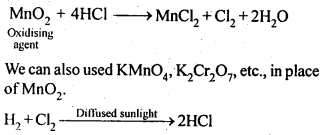
7.30. What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
Sol: N. Bartlett observed that PtF6 reacts with O2to give an compound O2+ [PtF6]–.
PtF6 (g) + O2 (g) ——–>O2+[PtF6]–
Since the first ionization enthalpy of Xe (1170 kJ mol-1 )is fairly close to that of 02 molecule (1175 kJ mol-1 ), he thought that PtF6 should also oxidise Xe to Xe+. This inspired Bartlett to carryout the reaction between Xe and PtF6. When PtF6 and Xe were made to react, a rapid reaction took place and a red solid, Xe+[PtF6]– was obtained.![]()
7.31. What are the oxidation states of phosphorus in the following: –
(i) H3PO3 (ii)PCl3
(iii) Ca3P2(iv)Na3PO4
(v) POF3
Sol: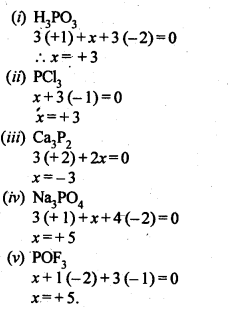
7.32. Write balanced equations for the following:
(i) NaCl is heated witlrsulphuric acid in the presence of MnO2
(ii) Chlorine gas is passed into a solution of Nal in water.
Sol: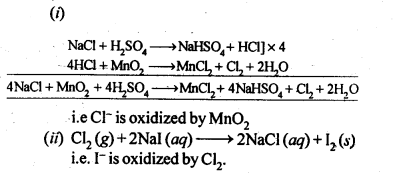
7.33. How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Sol: XeF2, XeF4 and XeF6 are obtained by direct reaction between Xe and F2 as follows: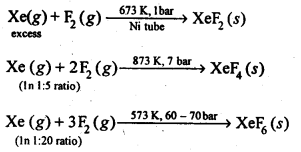
7.34. With which neutral molecule is ClO– isoelectronic? Is this molecule Lewis acid or base ? (Pb. Board 2009)
Sol: ClO– has (17 + 8 + 1) = 26 electrons. It is iso-electronic with two neutral molecules.
Oxygen difluoride (OF2) : 8 + 18 = 26 electrons
Chlorine fluoride (ClF) : 17 + 9 = 26 electrons
Out of these, ClF can act as Lewis base. The atom chlorine has three lone electron pairs which it donates to form compounds like ClF3, ClF5 and ClF7.
7.35. How are XeO3 and XeOF4prepared?
Sol: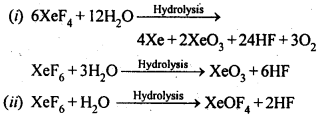
7.36. Arrange the following in the order of property indicated for each set: –
(i) F2 , Cl2 , Br2 , I2 – increasing bond dissociation enthalpy.
(ii) HF, HCI, HBr, HI – increasing acid . strength.
(iii) NH3, PH3, AsH3, SbH3, BiH3 – increasing Sol. base strength.
Sol: (i) Bond dissociation enthalpy decreases as the bond distance increases from F2 to I2 due to increase in the size of the atom, on moving from F to I.
F – F bond dissociation enthalpy is smaller then the Cl – Cl and even smaller than Br – Br. This is because F atom is very small and have large electron-electron repulsion among the lone pairs of electrons in F2 molecule where they are much closer to each other than in case of Cl2. The increasing order of bond dissociation enthalphy is I, < F2 < Br2 < Cl2
(ii) Acid strength of HF, HCI, HBr and HI depends upon their bond dissociation enthalpies. Since the bond dissociation enthalpy of H – X bond decreases from H – F to H-l as the size of atom increases from F to I.
Thus, the acid strength order is HF < HCI < HBr < HI
The weak acidic strength of HF is also due to H-bonding due to which release of H+ becomes difficult.
(iii) NH3, PH3, ASH3, SbH3 and BiH3 behaves as Lewis bases due to the presence of lone pair of electrons on the central atom. As we move from N to Bi, size of atom increases. Electron density on central atom decreases and hence the basic strength decreases from NH3 to BiH3. Thus basic strength order is BiH3<SbH3<AsH3<PH3<NH3
7.37. Which one of the following does not exist ?
(i)XeOF4 (ii)NeF2
(iii)XeF4 (iv)XeF6
Sol: NeF2 does not exist. This is because the sum of first and second ionization enthalpies of Ne are much higher than those of Xe. Consequently, F2 can oxidise Xe to Xe2+ but cannot oxidise Ne to Ne2+.
7.38. Give the formula and describe the structure of a noble gas species which is isostructural with: (i) ICI4– (ii) IBr2– (iii) Br03–
Ans: (i) ICI4–: In ICI4–, central atom I has seven valence electrons and one due to negative charge. Four out of these 8 electrons are utilized in forming four single bonds with four Cl atoms. Four remaining electrons constitutes the two lone pairs. It is arranged in square planar structure. ICI4– has 36 valence electrons. A noble gas species having 36 valence electrons is XeF4 (8 + 4 x 7 = 36). XeF4 is also square planar.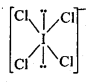
(ii) IBr2–: In IBr2–, central atom I has eight electrons. Two of these are utilized in forming two single bonds with two Br atom. Six remaining electrons constitutes three lone pairs. It is arranged in linear structure.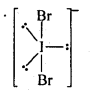
IBr2– has 22 valence electrons. A noble gas species having 22 valence electrons is XeF2 (8+2 x 7=22).
XeF2 is also linear.
(iii) In Br03– ion the central Br atom has 8 valence electrons (7 +1). Out of these, it shares 4 with two atoms of O forming Br = O bonds. Out of the remaining four .electrons, 2 are donated to the third O atom which accounts for its negative charge. The remaining 2 electrons constitute one lone pair. In order to minimise the force of repulsion, the structure of Br03– ion must be pyramidal. Br03– ion has (7 + 3 x 6 + 1) = 26 valence electrons and is isoelectronic as well as iso-structural with noble gas species Xe03 which has also 26(8 + 3 x 6) electrons.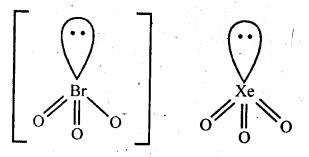
7.39. Why do noble gases have comparatively large atomic size?
Sol: The members of the noble gas family have comparatively large atomic size as compared to rest of the members present in the same period. Actually, for these elements, van der Waals’ radii are considered while for rest of the elements either covalent radii or metallic radii are taken into account. Since van der Waals’ radii arise simply due to van der Waals’ forces of attraction, these are expected to have comparatively large magnitude.
7.40. List the uses of neoirand argon gases.
Sol: Uses of Neon
Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes. Glow’of different colours ‘neon signs’ can be produced by mixing neon with other gases. Neon bulbs and used in botanical gardens and in green’ houses
DR OMAR CLASSES BARRA 3, MOBILE 9450149685
The group 16 elements of modern periodic
table consist of 5 elements oxygen, sulphur, selenium, tellurium and polonium.
The elements in this group are also known as the chalcogens or the ore-forming
elements because many elements can be extracted from the sulphide or oxide
ores.
Oxygen is abundantly found on the earth.
Estimating the proportions of different types of atoms found in the universe,
oxygen was claimed as the fourth abundant element after hydrogen, helium, and
neon. It constitutes about 89% of water, 46% of the earth crust and 20 % of the
air.
|
Period |
Element |
Symbol |
Atomic Number |
Electronic Configuration |
|
2 |
O |
8 |
[He]
2s2 2p4 |
|
|
3 |
S |
16 |
[Ne]
3s2 3p4 |
|
|
4 |
Se |
34 |
[Ar]
3d10 4s2 4p4 |
|
|
5 |
Te |
52 |
[Kr]
4d10 5s2 5p4 |
|
|
6 |
Po |
84 |
[Xe]4f14 5d10 6s2 6p4 |
Oxygen:
The chemical symbol for oxygen is given as
O. It is a colourless and odourless gas used in the respiration process by
humans, which is converted into carbon dioxide. Oxygen exists as a diatomic
molecule (O2). Oxygen is also found as a triatomic molecule (O3) in
traces, it is known as ozone.
DR OMAR CLASSES BARRA 3, MOBILE 9450149685
Oxygen combines readily with many elements.
During the combination with some elements, the evolution of heat energy takes
place, this process is known as combustion..
Sulphur:
Sulphur is denoted by the symbol S. It is a
non-metal which ranks ninth on the basis of cosmic abundance. About 1 atom in
every 20,000-30,000 atoms is a sulphur atom. Sulphur is found in the combined
state as well as in the free state. About 0.09 % of sulphur is found in
seawater in the form of sulphates. The meteorite contains 12 % of sulphur, a
large amount of sulphur is found from the underground deposits of pure sulphur
present in dome-like structures. Here sulphur is formed by the action of
anaerobic bacteria on the sulphate minerals such as gypsum.
Selenium:
Selenium is rarer than oxygen or sulphur.
It is found in the free state as well as in the combined state with heavy
metals (as lead, silver, or mercury) in a few minerals. Grey metallic form of
the selenium is the most stable form of the element under normal conditions.
Tellurium:
Tellurium is a chemical element having
atomic number 52 and has the properties between metals and non-metals. It is
one of the rarest stable elements found in the earth’s crust. It is often found
in a free state and in compounds with elements such as copper, lead, silver or
gold.
Polonium:
It is the rarest element among the group 16
elements. It is a radioactive element. Polonium is sometimes used in scientific
applications for alpha radiation.
The group 16 elements, also known as
the chalcogens have
6 valence electrons, and hence they can achieve noble gas configuration
either by gaining 2 electrons or by sharing two electrons i.e., by forming M2- ions,
or forming two covalent bonds. The oxidation state of elements is defined as
the number of electrons gained or lost to form a bond and its sign is the
indication of ionic charge on the element. Oxygen is the most electronegative
element after fluorine. The difference in electronegativity between metals and
oxygen is generally very high. We know that ionic bond is formed when the
difference in electronegativity of any two elements is very high. Thus, metals
and oxygen combine to form a metal oxide with oxygen attaining O2- form,
so the oxidation state of oxygen is mostly (-II).
Electronegativity of chalcogens decreases
down the group in the periodic
table. Other chalcogen elements like sulphur, selenium, and tellurium
react with a more electronegative element of group 1, 2 and lanthanides i.e.,
the uppermost elements of group 1, 2 and lanthanides to form sulphides,
selenides, and tellurides. These compounds are the most stable compounds formed
by these elements. Compounds formed above can be denoted as S2-, Se2-, and
Te2-. The electronegativity difference between the compounds
(sulphides, selenides, and tellurides) shows that they are on the borderline of
50% ionic and 50% covalent characters as in the case of PCl5.
Phosphorus pentachloride is sometimes covalent in the solid state but is ionic
in the aqueous state.
The chalcogen group can also share their
two electrons with another element to form two covalent bonds for example
l}H_2O, F_2O, Cl_2O, H_2S ~and ~SCl_2
. In the given examples
the chalcogen elements have the least electronegativity. As we can see in the
case of
(the electronegativity of chlorine = 3.5 and
sulphur =2.5) and the oxidation state of sulphur is (+II).
Electronic
configuration and Oxidation states (Unstable states are written in parentheses)
Element
|
Configuration
|
Oxidation
States
|
|
O |
[He]2s2 2p4 |
-II,
(-I) |
|
S |
[Ne]3s2 3p4 |
-II,
(II), IV, VI |
|
Se |
[Ar]3d10 4s2 4p4 |
(-II),
II, IV, VI |
|
Te |
[Kr]4d10 5s2 5p4 |
II, IV,
VI |
|
Po |
[Xe]4f14 5d10 6s2 6p4 |
II,
IV |
Oxygen and sulphur have only s and p
electrons, whereas the Se, Te, and Po have d electrons. The filling of d shell
makes the atom smaller and hence the electrons are tightly packed. Because of
this reason, Se cannot acquire the highest oxidation state of (+VI). Whenever,
S is oxidized by
(Se
+IV). Some elements of group 16 like S, Se, and Te can attain oxidation states
of IV and VI, which is more stable than the +II state. This is just the brief
layout of oxidation states of group 16 elements.
Group 16 elements in the modern periodic
table are known as the oxygen family.
The first four elements of this group are nonmetals, and they are termed as
‘the chalcogens’ or they can also be referred as ore-forming elements as most
of the metal ores are oxides or sulfides. This group consists of oxygen (O),
sulfur (S), selenium (Se), tellurium (Te) and polonium (Po) (a radioactive
element). These elements exist in free as well as in combined state.
In the electronic configuration, the word
‘electronic’ means electron and ‘configuration’ means an arrangement of
something. It depicts the arrangement of electrons in the orbital shells and
subshells. Before starting with filling the electron diagram we should know
some basic rules for the same. It is compulsory to fill the lowest energy level
first and then we need to proceed towards the higher energy levels. Pauli’s
exclusion principle, Hund’s rule, and the Aufbau’s principle should be
considered during the arrangement of electrons. Pauli’s exclusion principle
states that any two electrons in an atom cannot have the same four quantum numbers (n,
l, m, s). The starting three can be same but the fourth one (s) can never be
the same. According to the Hund’s rule, when electrons are arranged in
orbitals, then similar energy orbitals first occupy one electron in each of
them, then only the electrons can start pairing with other electrons in a
half-filled orbital. Lastly, according to the Aufbau’s principle, the electron
will occupy the lowest energy level first. Electronic configuration of
DR OMAR CLASSES BARRA 3, MOBILE 9450149685
chemical elements in the modern periodic
table can be written by considering the above principle and rules.
Atomic orbital diagonal
rule
Now that we know all the principles and
rules, we can write the electronic configuration of an element. As we are
reading about chalcogen elements, so we will start with oxygen. The atomic
number of oxygen is 8. In this case, first we will fill the 1s orbital with two
electrons (as it is the lowest energy level), and then we will fill the 2s and
2p orbital with 2 and 4 electrons respectively. So its electronic configuration
can be written as 1s2, 2s2, 2p4. Neil Bohr said that the electronic configurations of the
elements of the same group are similar to each other. So by noting this point,
we can give the configuration of other elements as shown in the table:
Atomic
number
|
Element
|
Configuration
|
|
8 |
O |
[He]2s2 2p4 |
|
16 |
S |
[Ne]3s2 3p4 |
|
34 |
Se |
[Ar]3d10 4s2 4p4 |
|
52 |
Te |
[Kr]4d10 5s2 5p4 |
|
84 |
Po |
[Xe]4f14 5d10 6s2 6p4 |
Looking at the table we can say that the
number of valence electrons of elements of chalcogen elements is 6. The symbol
of elements written in the square bracket is the nearest noble gas elements.
Now, we can say that the general electronic configuration of group 16 elements
is ns2np4.
DR OMAR CLASSES BARRA 3, MOBILE 9450149685
What are Halogens?
The
halogens are the elements that form group 17 of the periodic table. They are
reactive nonmetals and include fluorine, chlorine, bromine, and iodine.
Halogens are highly reactive non-metals.
These elements greatly resemble in property with each other. Group 17 elements
are collectively called as halogens (In Greek: halo means salt and genes mean
producing, so collectively salt producing) and it consists of fluorine,
chlorine, bromine, iodine, and astatine.
The similarity to this extent is not found
in other groups of the periodic table. They have a regular gradation in the
physical and chemical properties. Astatine
is the only radioactive element in the group. They have seven
electrons in their outermost shell (ns2np5) and
are short of one electron from the configuration of the nearest noble gas. The
chemical properties and reactivity of an element are determined by the
oxidation state exhibited by them.
Oxidation
State
- All the elements of the halogen family
exhibit -1 oxidation state. However, elements such as chlorine, bromine,
and iodine also show +1, +3, +5 and +7 state.
- This higher oxidation state of chlorine,
bromine, and iodine is
realized when these halogens are in combination with small and highly
electronegative atoms of fluorine and oxygen.
- The oxides and oxoacids of chlorine and
bromine have +4 and +6 states. There are no valence shells d orbitals in
fluorine atom and therefore it cannot expand its octet.
- Fluorine being the most electronegative
element exhibits only -1 oxidation state.
Chemical
Properties
1. Halogens are highly reactive, they react with metals and
non-metals in order to form halides. Their reactivity decreases as we move down
the group. Halogens have strong oxidizing properties. F2 is
the strongest oxidizing halogen. It easily oxidizes other halide
ions present in solution or in the solid phase. In general, a halogen oxidizes
halide ion which is of higher atomic number. For example:
F2 + 2X– →
2F– + X2 (X
= Cl, Br or I)
From the standard
electrode potential, the decreasing oxidizing ability of halogen can be easily
observed.
2. The relative oxidizing nature of
halogens can be illustrated by their reactions with water. Fluorine oxidizes
water to oxygen. Whereas chlorine and bromine react with water in order to form
respective hydrohalic and hypohalous acids. Iodine reacts with water in a
non-spontaneous way. I– can be oxidized by water
in the acidic medium. For example:
4 I– (aq)
+ 4H+ (aq) + O2 (g)
→ 2I2(s) +
2H2O (l)
General
Characteristics of Halogen Family:
1. Electronic Configuration of Halogen
Family
Members of the halogen family have seven valence electrons, that
is, they have seven electrons in their outermost orbit. Thus, they are one
electron short of the nearest noble gas configuration. The general
configuration of the halogen family is given as ns2np5.
2. Atomic and Ionic Radii of Halogen Family
The members of group 17 have the smallest atomic radii in their
respective periods. This is attributed to the fact that they have a maximum
effective nuclear charge. Atomic and ionic radii increase from top to bottom in
a group thus, it increases from fluorine to iodine due to the increasing number
of quantum shells.
DR OMAR CLASSES BARRA 3, MOBILE 9450149685
3. Ionisation Enthalpy of Halogen Family
Members of group 17 have very little or no tendency to lose an
electron. Thus, they have a very high value of ionisation enthalpy. Ionisation
enthalpy decreases from top to bottom in the group due to the increase in
atomic size.
4. Electron Gain Enthalpy of Halogen Family
The atoms of group 17 elements are only one electron short of
attaining stable noble gas configurations. Thus, these elements have a maximum
negative electron gain enthalpy in the corresponding periods. Electron
gain enthalpy of
these elements becomes less negative as we move down the group due to the
increase in atomic size. However, chlorine has a more negative value of
electron gain enthalpy with respect to fluorine.
1.
Electronegativity of Halogen Family
The elements of group 17
have a very high value of electronegativity. The electronegativity decreases
down the group due to the decrease in effective nuclear charge. Thus, fluorine
is the most electronegative element.
Occurrence of Group 18
Elements
Group 18 elements are called as the noble
gases or inert gases due to completely filled valence shells which makes them
highly stable. They occupy the last column of the periodic table. Group 18 of
the periodic table consists of six elements:
DR OMAR CLASSES BARRA 3, MOBILE
9450149685
The existence of helium was first claimed
in the year 1868. Radioactive elements were used to establish the existence of
this element. It is found in the atmosphere and in earth’s crust in small
quantities.
Noble Gases – Group 18 Elements
Argon, the second member of group 18
elements, gets its name from a Greek word which means inert representing the
chemical nature of argon. Argon found in nature makes up to 0.947% of dry air.
It is the most abundant noble gas found in dry air. The concentration of argon
above the sea level is slightly more. The solubility of argon in water is more
than nitrogen which makes it available in sea and river water. This is the
reason behind the presence of argon gas in the gas-bladder of fishes. It is
found in the free state in plants and blood cells of animals.
Neon was discovered by Morris W.Travers and
Sir William Ramsay in the year 1898. This noble gas is widely distributed in
the universe and it is the fifth most abundant element in the universe. Neon
can be created at high temperatures by thermonuclear reaction between oxygen
and carbon which occurs in star cores. Many stars have stable isotopes of neon
due to this reaction.
Krypton was discovered by Sir William
Ramsay and Morris Travers in the year 1898. In earth’s atmosphere, the
concentration of Krypton is observed to be around 1ppm. Krypton is a
colourless, odourless and tasteless gas which is often used in fluorescent
lamps in combination with other rare gases. The percentage of krypton in space
depends on meteoric activities and solar winds.
Xenon is the second last member of group 18
of the modern periodic table having the atomic number of 54. In the earth’s
atmosphere xenon is found in traces. Some mineral springs emit xenon.
Commercially it is obtained as a by-product of separation of air into oxygen
and nitrogen. It has 8 naturally occurring stable isotopes and more than 40
unstable isotopes that undergo radioactive decay. The atmosphere of planet
Jupiter contains xenon in high quantity.
Radon was discovered by Friedrich Ernst
Dorn in the year 1900. Its atomic number is 86 and has a symbol Rn. Radon is
found in uranium rocks, phosphate rocks, metamorphic and igneous rocks due to
the radioactive decay of radium-226. Industrially radon is obtained as a
by-product the processing of uraniferous ores.
Group 18 Elements - Characteristics of Noble
Gases
Members of group 18 in the modern periodic table are known as the noble gases.
They are:
·
Helium (He)
·
Neon (Ne)
·
Argon (Ar)
·
Krypton (Kr)
·
Xenon (Xe)
·
Radon (Rn)
DR OMAR CLASSES BARRA 3, MOBILE
9450149685
The members of the group have eight electrons in their outermost orbit (except helium which has two electrons). Thus, they have a stable configuration. Group 18 elements are gases and chemically unreactive, which means they don’t form many compounds. Thus, the elements are known as inert gases. Like the other group elements, noble gas elements also exhibit trends in their physical and chemical properties. The general characteristics of noble gases are discussed below.
Neon Electronic
Configuration
Characteristics of Group 18 Elements:
1.
Electronic configuration of noble gases:
Members of group 18 have eight valence electrons, i.e., they have eight electrons in their outermost orbit (except helium). Thus, they exhibit a stable octet configuration. But helium exhibits a duplet configuration. The general configuration of the noble gas family is given as ns2np6 (except helium which has 1s2).
2.
Atomic radii of noble gases:
The members of group 18 have very small atomic radii. Atomic radii of noble gases increase down the group with an increase in atomic number due to the addition of new shells.
3.
Ionization enthalpy of noble gases:
Noble gases have eight valence electrons, that is, they have eight electrons in their outermost orbit (except helium). Thus, they exhibit stable octet or duplet configuration. Hence, the elements of group 18 exhibit very high ionization enthalpies. Ionization enthalpy of noble gases decreases down the group due to the increase in their atomic size.
4.
Electron gain enthalpy of noble gases:
Members of the group have eight valence electrons (except helium). Thus, they exhibit stable octet or duplet configuration. Hence, elements of group 18 exhibit very large positive values of electron gain enthalpy.
DR OMAR CLASSES BARRA 3, MOBILE 9450149685


No comments:
Post a Comment