DR OMAR CLASSES KAMPUR
Access Answers to NCERT Class 10 Science Chapter 1 – Chemical Reactions and Equations
In-text questions set 1 Page number – 6
1. Why should a magnesium ribbon be cleaned before burning in air?
Solution:
Magnesium ribbon should be cleaned before burning in air because Magnesium metal reacts with the atmospheric oxygen and forms Magnesium Oxide (MgO) layer which is a very stable compound. In order to prevent further reactions with Oxygen, it is therefore necessary to clean the ribbon by to remove the layer of MgO.
2. Write a balanced equation for the following chemical reactions.
i) Hydrogen + Chloride —-> Hydrogen chloride
ii) Barium chloride + Aluminium sulphate —-> Barium sulphate + Aluminium chloride
iii) Sodium + Water —-> Sodium hydroxide + Hydrogen
Solution:
i) H2 + Cl2 → 2HCl
ii) 3BaCl2 + Al2(SO4)3 →3BaSO4 + 2AlCl3
iii) 2Na + 2H2O → 2NaOH + H2
3. Write a balanced chemical equation with state symbols for the following reactions
i) Solutions of Barium chloride and Sodium sulphate in water react to give insoluble Barium sulphate and solution of Sodium chloride.
ii) Sodium hydroxide solution in water reacts with hydrochloric acid solution to produce Sodium chloride solution and water.
Solution:
i) BaCl2 + Na2SO4 → BaSO4 + 2NaCl
ii) NaOH + HCl → NaCl + H2O
In-text questions set 2 Page number – 10
1. A solution of a substance ‘X’ is used for whitewashing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Solution:
i) The substance ‘X’ which is used in whitewashing is quick lime or Calcium Oxide and its formula is CaO.
ii) CaO + H2O → Ca(OH)2
2. Why is the amount of gas collected in one of the test tubes in Activity 1.7 double of the amount collected in the other? Name this gas
Solution:
In activity 1.7, gas collected in one of the test tubes is double of the amount collected in the other because water gets hydrolysed to release H2 and O2 gas. Here, after electrolysis two molecules of Hydrogen and one molecule of oxygen gas is released, hence the amount of Hydrogen collected would be double than that of oxygen.
In-text questions set 3 Page number – 13
1. Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Solution:
When an iron nail dipped in the copper sulphate solution, iron displaces copper from the copper sulphate because iron is more reactive than copper. Therefore the colour of the copper sulphate solution changes. The reaction is:
Fe + CuSO4 → FeSO4 + Cu
2. Give an example of a double displacement reaction other than the one given in Activity 1.10.
Solution:
Reaction Between silver nitrate (AgNO3) and Sodium chloride (NaCl) is an example of double displacement reaction. During the reaction negative and positive ions trade positions as a result in the formation of white silver chloride precipitate. The chemical reaction is given below.
Ag+ + NO3– + Na+ + Cl– → AgCl + Na+ + NO3–
3. Identify the substances that are oxidized and that are reduced in the following equation.
i) 4Na(s) + O2(g) → 2Na2O(s)
ii) CuO(s) + H2(g) → Cu(s) + H2O(l)
Solution:
The Sodium (Na) in the first equation is getting oxidized with the addition of Oxygen (O2) and the Copper (Cu) in the second equation is reduced due to the addition of Hydrogen (H2)
Exercise Questions Page number – 14-16
1. Which of the statements about the reaction below are incorrect?
2PbO(s) + C(s) → 2Pb(s) + CO2(g)
(a) Lead is getting reduced
(b) Carbon Dioxide is getting oxidised
(c) Carbon is getting oxidised
(d) Lead oxide is getting reduced
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Solution:
(i) (a) and (b)
Explanation: (a) because Oxygen is being removed and (b) because the removed oxygen from Lead is added to the elemental Carbon.
2. Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a
- Combination reaction.
- Double displacement reaction.
- Decomposition reaction.
- Displacement reaction.
Solution:
Answer is 4. Displacement reaction.
Explanation: The Oxygen from the Ferrous oxide is getting displaced to the Aluminium metal to form Aluminium Oxide. In this reaction Aluminum is more reactive metal than Fe. Therefore Al will displace Fe from its oxide. This type of chemical reactions in which one of the elements displace another is called displacement reaction. Here less reactive metal is displaced by more reactive metal. Since one-time displacement is occurring, therefore, it is called a single displacement reaction.
3. What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.
- Hydrogen gas and Iron chloride are produced.
- Chlorine gas and Iron hydroxide are produced.
- No reaction takes place.
- Iron salt and water are produced.
Solution:
- Hydrogen gas and Iron chloride are produced.
Explanation: The Chlorine from Hydrogen chloride is displaced by the Iron fillings to undergo the following reaction.
2HCl + Fe → FeCl2 + H2
4. What is a balanced chemical equation? Why should a chemical equation be balanced?
Solution:
A balanced equation is the one in which number of different atoms on both the reactant and product sides are equal. Balancing chemical equation is necessary for the reaction should obey The Law of Conservation of mass. Balancing the chemical equation has no defined method and is purely a trial and error attempt.
c) Unbalanced:
BaCl2 + Al2(SO4)3 → AlCl3 + BaSO4
Balanced: 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4
(d) Unbalanced: K + H2O → KOH + H2
Balanced: 2K + 2H2O → 2KOH + H2
6. Balance the following chemical equations.
(a) HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O
(b) NaOH + H2SO4 → Na2SO4 + H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + HCl
Solution:
(a) 2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
(b) 2NaOH + H2SO4 → Na2SO4 + 2H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + 2HCl
7. Write the balanced chemical equation for the following reactions.
Calcium hydroxide + Carbon dioxide —-> Calcium carbonate + Water
Zinc + Silver nitrate —-> Zinc nitrate + Silver
Aluminium + Copper chloride —-> Aluminium chloride + Copper
Barium chloride + Potassium sulphate —-> Barium sulphate + Potassium chloride
Solution:
2Ca(OH)2 + 2CO2 → 2CaCO3 + 2H2O
Zn + 2AgNO3 → Zn(NO3)2 + 2Ag
2Al + 3CuCl2 → 2AlCl3 + 3Cu
BaCl2 + K2SO4 → BaSO4 + 2KCl
8. Write a balanced chemical equation for the following and identify the type of reaction of each case
KBr + BaI2 → KI + BaBr2
ZnCO3 → ZnO + CO2
H2 + Cl → HCl
Mg + HCl → MgCl2 + H2
Solution:
2KBr + BaI2 → 2KI + BaBr2 (Double Displacement Reaction)
ZnCO3 → ZnO + CO2 (Decomposition Reaction)
H2 + Cl → 2HCl (Combination Reaction)
Mg + 2HCl → MgCl2 + H2 (Displacement Reaction)
9. What is meant by exothermic and endothermic reactions? Give examples.
Solution:
An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat.(Example: Photosynthesis, melting of ice, evaporation). Conversely, an exothermic reaction is one in which energy is released from the system into the surroundings. (Example: Explosions, concrete setting, nuclear fission and fusion).
10. Why is respiration considered to be an exothermic reaction?
Solution:
For the survival of life, we require energy. We obtain this energy from the food we eat. The food molecules, through the process of digestion, is broken down into a simpler molecule like glucose. These substances come in contact with the Oxygen present in our body cells to form Carbon dioxide and water along with a certain amount of energy (Respiration process). Since the energy is in the form of heat (that maintains our body temperature) the respiration is considered to be an exothermic reaction. The reaction taking place is:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
11. Why are decomposition reactions called the opposite of Combination reactions? Write equations for decomposition reactions.
Solution:
Combination reaction is said to be the reaction between two or more molecules to form a larger molecule; whereas the decomposition reaction is defined as the splitting of larger molecules into two or more smaller molecules. This essentially explains that the decomposition reaction is the opposite of the combination reaction.
In most of the cases the decomposition reaction is endothermic since heat from the surrounding or induced heat is used to break the bonds of the larger molecule. Few examples of decomposition reactions are:
ZnCO3 → ZnO + CO2
CaCO3 + Energy → CaO + CO2
2HgO → 2Hg + O2
12. Write one equation each for decomposition reactions in which energy is supplied in the form of heat, light or electricity.
Solution:
(a) Thermal decomposition reaction (Thermolysis)
Decomposition of potassium chlorate: When heated strongly, potassium chlorate decomposes into potassium chloride and oxygen. This reaction is used for the preparation of oxygen.
2KClO3 + Heat → 2KCl + 3O2
(b) Electrolytic decomposition reaction (Electrolysis)
Decomposition of sodium chloride: On passing electricity through molten sodium chloride, it decomposes into sodium and chlorine.
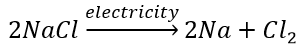
(c) Photodecomposition reaction (Photolysis)
Decomposition of hydrogen peroxide: In the presence of light, hydrogen peroxide decomposes into water and oxygen.
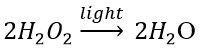
13. What is the difference between displacement and double displacement reactions? Write relevant equations for the above.
Solution:
A displacement reaction is the one when a more reactive substance displaces a less reactive one from its salt solution whereas a double displacement reaction is the one where a mutual exchange of ions happens between two compounds.
In a displacement reaction, only a single displacement takes place whereas in the double displacement reaction, as the name suggests two displacement takes place between the molecules.
Example:
Displacement reaction
Mg + 2HCl → MgCl2 + H2
Double displacement reaction
2KBr + BaI2 → 2KI + BaBr2
14. In the refining of Silver, the recovery of silver from Silver nitrate solution involves displacement reaction by Copper metal. Write down the reaction involved.
Solution:
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
15. What do you mean by a precipitation reaction? Explain by giving examples.
Solution:
When two solutions containing soluble salts are combined, a double displacement reaction takes place in which the ions are exchanged between the compounds. When one of such compounds formed is in solid form (that is insoluble in aqua) then it settles down at the bottom of the container. This solid is known as the precipitate and the respective reaction is termed as the precipitation reaction. Few examples of precipitation reactions are:
CdSO4(aq) + K2S(aq) → CdS(s) + K2SO4(aq)
2NaOH(aq) + MgCl2(aq) → 2NaCl(aq) + Mg(OH)2(s)
16. Explain the following in terms of gain of oxygen with two examples each.
(a) Oxidation
(b) Reduction
Solution:
(a) In a chemical reaction, when the oxygen is added to the element to form its respective oxide it is the element being oxidised. Example:
4Na(s) + O2(g) → 2Na2O(s)
H2S + O2 → H2O + SO2
(b) In a chemical reaction, when the oxygen is being removed from the compound then it is said to be reduced. Example:
CuO(s) + H2(g) → Cu(s) + H2O(l)
2HgO → 2Hg + O2
17. A shiny brown coloured element ‘X’ on heating in the air becomes black in colour. Name the element ‘X’ and the black coloured compound formed.
Solution:
The shiny brown coloured element is the Copper metal (Cu). When the metal is heated in air, it reacts with atmospheric oxygen to form copper oxide. Hence, the black coloured compound is the copper oxide.
2Cu(s) + O2(g) → 2CuO(s)
18) Why do we apply paint on iron articles?
Solution:
Iron articles are painted to prevent them from rusting. When left unpainted, the metal surface comes in contact with the atmospheric oxygen and in the presence of moisture it from Iron(III) oxide. But if painted the surface does not come in contact with moisture and air thus preventing Rusting.
19) Oil and Fat containing food items are flushed with Nitrogen. Why?
Solution:
The main purpose of flushing Nitrogen into food packets that contain oil and fat items is to prevent Rancidity which occurs when the oil or fat reacts with the oxygen letting out an unpleasant smell and taste. Therefore, by flushing Nitrogen, an unreactive surrounding is created thus preventing rancidity.
20) Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Solution:
(a) Corrosion is a process where a refined metal is oxidised by atmospheric oxygen to form a more stable compound such as oxides. The metal gradually degrades during the corrosion process. Rusting of iron is a good example of corrosion where the iron is converted to Iron oxide. Millions of dollars are spent annually in preventing rusting from bridges and other monuments.
(b) The condition produced by the aerial oxidation of the oil and fat present in the food material that produces an unpleasant taste and smell. The rancidity is retarded when the food is kept inside the refrigerator since the low temperature does not promote the oxidation reaction.
DR OMAR CLASSES BARRA KANPUR
CBSE Class 10 Science Notes Chapter 2 Acids Bases And Salts:
Introduction to Acids, Bases and Salts
Classification of matter
On
the basis of
a) composition – elements, compounds and mixtures
b) state – solids, liquids and gases
c) solubility – suspensions, colloids and solutions
Types
of mixtures – homogeneous and heterogeneous
Types of compounds – covalent and ionic
To
know more about Classification of Matter,
What Is an Acid and a Base?
Ionisable and non-ionisable compounds
An
ionisable compound when dissolved in water or in its molten state, dissociates
into ions almost entirely. Example: NaCl, HCl, KOH, etc.
A non-ionisable compound does not dissociate into ions when dissolved in
water or in its molten state. Example: glucose, acetone, etc.
Arrhenius theory of acids
and bases
Arrhenius
acid – when dissolved in water, dissociates to give H+ (aq) or H3O+ ion.
Arrhenius base – when dissolved in water, dissociates to give OH− ion.
Examples
Acids
- Hydrochloric
acid (HCl)
- Sulphuric
acid (H2SO4)
- Nitric
acid (HNO3)
Bases
- Sodium
hydroxide (NaOH)
- Potassium
hydroxide (KOH)
- Calcium
hydroxide (Ca(OH)2)
To
know more about Arrhenius Theory,
Bronsted Lowry theory
A
Bronsted acid is an H+ (aq)
ion donor.
A Bronsted base is an H+ (aq) ion acceptor.
Example
In
the reaction: HCl (aq) + NH3 (aq) → NH+4(aq) + Cl− (aq)
HCl – Bronsted acid and Cl− : its conjugate acid
NH3 – Bronsted base and
NH+4 : its conjugate
acid
To
know more about Bronsted Lowry Theory,
Physical test
Given are
two possible physical tests to identify an acid or a base.
a. Taste
An
acid tastes sour whereas a base tastes bitter.
The method of taste is not advised as an acid or a base could be contaminated
or corrosive.
b. Effect on indicators
by acids and bases
An
indicator is a chemical substance which shows a change in its physical
properties, mainly colour or odour when brought in contact with an acid or a
base.
Below mentioned are commonly used indicators and the different colours they
exhibit:
a) Litmus
In a neutral solution – purple
In acidic solution – red
In basic solution – blue
Litmus
is also available as strips of paper in two variants – red litmus and blue litmus.
An acid turns a moist blue litmus paper to red.
A base turns a moist red litmus paper to blue.
b) Methyl orange
In a neutral solution – orange
In acidic solution – red
In basic solution – yellow
c) Phenolphthalein
In a neutral solution – colourless
In acidic solution – remains colourless
In basic solution – pink
Acid-Base Reactions
Reactions of acids and
bases
a) Reaction of acids and
bases with metals
Acid +
active metal → salt + hydrogen + heat
2HCl
+ Mg → MgCl2 + H2 (↑)
Base +
metal → salt + hydrogen + heat
2NaOH
+ Zn → Na2ZnO2 + H2 (↑)
A
more reactive metal displaces the less reactive metal from its base.
2Na
+ Mg (OH) 2 → 2NaOH + Mg
b) Reaction of acids with
metal carbonates and bicarbonates
Acid +
metal carbonate or bicarbonate → salt + water + carbon dioxide.
2HCl
+ CaCO3 → CaCl2 + H2O + CO2
H2SO4 + Mg (HCO3)2 → MgSO4 + 2H2O + 2CO2
Effervescence
indicates liberation of CO2 gas.
c) Neutralisation
reaction
1.
Reaction of metal oxides and hydroxides with acids
Metal oxides or metal hydroxides are basic in nature.
Acid + base → salt + water + heat
H2SO4 + MgO → MgSO4 + H2O
2HCl + Mg (OH) 2 → MgCl2 + 2H2O
2.
Reaction of non-metal oxides with bases
Non-metal oxides
are acidic in nature
Base + Nonmetal oxide → salt + water + heat
2NaOH
+ CO2→ Na2CO3 + H2O
To
know more about Properties of Acids and Base
Water
Acids and bases in water
When
added to water, acids and bases dissociate into their respective ions and help
in conducting electricity.
Difference between a base
and an alkali
Base:
- Bases
undergo neutralisation reaction with acids.
- They are
comprised of metal oxides, metal hydroxides, metal carbonates and metal
bicarbonates.
- Most of
them are insoluble in water.
Alkali:
- An alkali
is an aqueous solution of a base, (mainly metallic hydroxides).
- It
dissolves in water and dissociates to give OH− ion.
- All
alkalis are bases, but not all bases are alkalis.
To
know more Difference between a base and an alkali,
Hydronium ion
Hydronium
ion is formed when a hydrogen ion accepts a lone pair of electrons from the
oxygen atom of a water molecule, forming a coordinate covalent bond.
Dilution
Dilution
is the process of reducing the concentration of a solution by adding more
solvent (usually water) to it.
It is a highly exothermic process.
To dilute acid, the acid must be added to water and not the other way round.
Strength of acids and
bases
Strong acid or base: When all molecules of a given amount of
an acid or a base dissociate completely in water to furnish their respective
ions, H+(aq) for acid and OH−(aq) for base).
Weak acid or base: When only a few of the molecules
of a given amount of an acid or a base dissociate in water to furnish their
respective ions, H+(aq) for acid and OH−(aq) for base).
Dilute acid: contains less number of H+(aq) ions per unit
volume.
Concentrated acid: contains more number of H+(aq) ions per unit
volume.
Universal indicator
A universal
indicator has a pH range from 0 to 14 that indicates the acidity or
alkalinity of a solution.
A neutral solution has pH=7
pH
pH=−log10[H+]
In pure water, [H+]=[OH−]=10−7 mol/L. Hence, the
pH of pure water is 7.
The pH scale ranges from 0 to 14.
If pH < 7 → acidic solution
If pH > 7→ basic solution
pH
scale
Importance of pH in
everyday life
1. pH sensitivity of
plants and animals
Plants
and animals are sensitive to pH. Crucial life processes such as digestion of
food, functions of enzymes and hormones happen at a certain pH value.
2. pH of a soil
The
pH of a soil optimal for the growth of plants or crops is 6.5 to 7.0.
3. pH in the digestive
system
The
process of digestion happens at a specific pH in our stomach which is 1.5 to 4.
The pH of the interaction of enzymes, while food is being digested, is
influenced by HCl in our stomach.
4. pH in tooth decay
Tooth
decay happens when the teeth are exposed to an acidic environment
of pH 5.5 and below.
5. pH of self-defence by
animals and plants
Acidic
substances are used by animals and plants as a self-defence mechanism. For
example, bee and plants like nettle secrete a highly acidic substance for
self-defence. These secreted acidic substances have a specific pH.
To
know more about pH,
Manufacture of Acids and
Bases
Manufacture of acids and
bases
a)
Nonmetal oxide + water → acid
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
4NO2(g) + 2H2O(l) + O2(g) → 4HNO3(aq)
Non-metal oxides
are thus referred to as acid anhydrides.
b)
Hydrogen + halogen → acid
H2(g) + Cl2(g) → 2HCl(g)
HCl(g) + H2O(l) → HCl(aq)
c)
Metallic salt + conc. sulphuric acid → salt + more volatile acid
2NaCl(aq) + H2SO4(aq) → Na2SO4(aq) + 2HCl(aq)
2KNO3(aq) + H2SO4(aq) → K2SO4(aq) + 2HNO3(aq)
d) Metal
+ oxygen → metallic oxide (base)
4Na(s) + O2(g) → 2Na2O(s)
2Mg(s) + O2(g) → 2MgO(s)
e)
Metal + water → base or alkali + hydrogen
Zn(s) + H2O(steam) → ZnO(s)+ H2(g)
f) Few
metallic oxides + water → alkali
Na2O(s) + H2O(l) → 2NaOH(aq)
g) Ammonia
+ water → ammonium hydroxide
NH3(g) + H2O(l) → NH4OH(aq)
Salts
Salts
A salt is a combination of an
anion of an acid and a cation of a base.
Examples – KCl, NaNO3 ,CaSO4, etc.
Salts are usually prepared by
the neutralisation reaction of an acid and a base.
Common salt
Sodium Chloride (NaCl) is
referred to as common salt because it’s used all over the world for cooking.
Family of salts
Salts having the same cation or
anion belong to the same family. For example, NaCl, KCl, LiCl.
pH of salts
A salt of a strong acid and a
strong base will be neutral in nature. pH = 7 (approx.).
A salt of a weak acid and a strong base will be basic in nature. pH > 7.
A salt of a strong acid and a weak base will be acidic in nature. pH < 7.
The pH of a salt of a weak acid and a weak base is determined by conducting a
pH test.
To know more about Salt,
Preparation of Sodium hydroxide
Chemical formula – NaOH
Also known as – caustic soda
Preparation
(Chlor-alkali process):
Electrolysis of brine (solution of common salt, NaCl) is carried out.
At anode: Cl2 is
released
At cathode: H2 is
released
Sodium hydroxide remains in the solution.
Bleaching powder
Chemical formula – Ca(OCl)Cl or
CaOCl2
Preparation – Ca(OH)2(aq)+Cl2(g)→CaOCl2(aq)+H2O(l)
On interaction with water –
bleaching powder releases chlorine which is responsible for bleaching action.
Baking soda
Chemical name – Sodium hydrogen
carbonate
Chemical formula – NaHCO3
Preparation
(Solvay process):
a. Limestone is heated: CaCO3→CaO+CO2
b. CO2 is
passed through a concentrated solution of sodium chloride and ammonia:
NaCl(aq)+NH3(g)+CO2(g)+H2O(l)→NaHCO3(aq)+NH4Cl(aq)
Uses:
1. Textile industry
2. Paper industry
3. Disinfectant
Washing soda
Chemical name – Sodium hydrogen
carbonate
Chemical formula – NaHCO3
Preparation
(Solvay process) –
a. Limestone is heated: CaCO3 → CaO + CO2
b. CO2 is
passed through a concentrated solution of sodium chloride and ammonia:
NaCl(aq) + NH3(g) + CO2(g) + H2O(l) →
NaHCO3(aq) +
NH4Cl(aq)
Uses
1. In glass, soap and paper industries
2. Softening of water
3. Domestic cleaner
Crystals of salts
Certain salts form crystals by
combining with a definite proportion of water. The water that combines with the
salt is called water of crystallisation.
Plaster of paris
Gypsum, CaSO4.2H2O (s) on heating at 100°C (373K) gives CaSO4. ½ H2O and 3/2
H2O
CaSO4. ½ H2O is
plaster of paris.
CaSO4. ½ H2O means two
formula units of CaSO4 share one
molecule of water.
Uses –
cast for healing fractures.
To know more about Plaster of
Paris, Baking Soda and Washing Soda,
Frequently Asked Questions on Acids,
Bases and Salts
When
dissolved in water, Arrhenius base is a compound that ……….?
An Arrhenius base is a compound
that increases the concentration of OH– ions
that are present when added to water.
A
solution X has a pH value of 2 and another solution Y has a pH value of 1. What
can be inferred regarding the difference in hydrogen ion concentration between
them?
pH is a measure of hydrogen ion
concentration in a solution. Higher the hydrogen ion concentration, lower is
the pH. Acids which give rise to more hydrogen ions are more acidic than the
acids which give less hydrogen ions. Thus, lower the pH, higher is the acidic
nature of the solution. Thus, Y has more hydrogen ion concentration than X.
Dilute
hydrochloric acid (HCl) reacts with metals to evolve __ gas along with the
formation of corresponding metal salt.
Acids react with metals to
produce the respective metal salt along with hydrogen gas. Therefore, when
hydrochloric acid (HCl) reacts with metals it evolve hydrogen gas along with
the formation of corresponding metal salt.
DR OMAR CLASSES BARRA KANPUR
NCERT Solutions for Class 10 Science Chapter 2
Acids, Bases and Salts
NCERT Solutions For Class 10 Science Chapter 2 Acids And
Bases: In this article, we will provide you with NCERT
Solutions For Class 10 Science Chapter 2 Acids And Bases. Having proper
knowledge of the theories, sufficient practice of the reactions, equations and
formulas, and solving questions from the NCERT Chemistry books are very
important if you want to score well in Science for Class 10 board exams as well
as JEE and NEET. Going through the step-wise solutions for every question too
is highly important to bag a good score.
Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts
NCERT
Solutions For Class 10 Science Chapter 2 Notes for Acids And Bases has
been provided by India’s topmost Chemistry teachers. Also in this article, you
will find the Step-wise explanation for each and every question. Going through
them will help you in getting a better understanding of how to solve
problems. Read on to find out NCERT Solutions
for Class 10 Science Chapter 2 Exercise and Extra
Questions.
NCERT
Solutions for Class 10 Science Chapter 2 Acids and Bases
Before
getting into the details of NCERT Solutions For Class 10 Science Chapter 2
Acids And Bases, let’s have an overview of topics & subtopics under
NCERT Solutions for Class
10 Science Chapter 2 Activities:
1.
Acids, Bases And Salts
2.
Understanding The Chemical Properties Of Acids And Bases
3.
What Do All Acids And All Bases Have In Common?
4.
How Strong Are Acid Or Base Solutions?
5.
More About Salts
Free
download NCERT
Solutions for Class 10 Science Chapter 2 Acids And Bases PDF in
Hindi Medium as well as in English Medium for CBSE, Uttarakhand, Bihar, MP
Board, Gujarat Board, and UP Board students, who are using NCERT Books based on
updated CBSE Syllabus for the session 2019-20.
NCERT Solutions for Class 10 Science Chapter
Page Number: 18
Question
1
You have been provided with three test tubes. One of them contains distilled
water and the other two contain an acidic solution and a basic solution,
respectively. If you are given only red litmus paper, how will you identify the
contents of each test tube ?
Answer:
(i) Put the red litmus paper in all the test tubes, turn by turn. The solution
which turns red litmus to blue will be a basic solution. The blue litmus paper
formed here can now be used to test the acidic solution.
(ii) Put the blue litmus paper obtained above in the remaining two test-tubes,
turn-by-turn. The solution which turns the blue litmus paper to red will be the
acidic solution.
(iii) The solution which has no effect on any litmus paper will be neutral and
hence it will be distilled water.
Page Number: 22
Question 1
Why should curd and sour substances not be kept in brass and copper vessels ?
Answer:
Curd and sour substances should not be kept in brass and copper vessels because
these and other sour food-stuffs contain acids which can react with the metal
of the vessel to form poisonous metal compounds which can cause food poisoning
and affect our health adversely.
Question 2
Which gas is usually liberated when an acid reacts with a metal ? Illustrate
with an example. How will you test for the presence of this gas ?
Answer:
(i) Hydrogen (H2)
gas is liberated when an acid reacts with a metal.
(ii) Illustration : Set up the apparatus as shown in the given figure. Take
some zinc granules in the test tube. Add about 5 mL dilute hydrochloric acid
slowly. Soon the reaction between zinc and hydrochloric acid starts and
hydrogen gas is evolved.
(iii) Test for H2 gas
:
H2 gas
is not soluble in water. When passed through soap solution, it gets trapped
into bubbles.
Bring a burning candle near the soap bubble filled with gas. The soap bubble
bursts and hydrogen gas burns with a pop sound.
Question 3
Metal compound A reacts with dilute hydrochloric acid to produce effervescence.
The gas evolved extinguishes a burning candle. Write a balanced chemical
equation for the reaction if one of the compounds formed is calcium chloride.
Answer:
As the end product is calcium chloride and the gas formed is carbon dioxide,
the metal compound A must be calcium carbonate. Therefore, the reaction between
calcium carbonate and hydrochloric acid is
Page Number: 25
Question 1
Why do HCl, HNO3,
etc show acidic characters in aqueous solutions while solutions of compounds
like alcohol and glucose do not show acidic character ?
Answer:
H+ ions
in aqueous solution are responsible for acidic character. HCl, HNO3, etc. give H+ ions in water while alcohol and
glucose do not give H+ ion
in water. Therefore, alcohol and glucose do not show acidic character.
Question 2
Why does an aqueous solution of an acid conduct electricity ?
Answer:
The aqueous solution of an acid conducts electricity due to the presence of
charged particles called ions in it.
Question 3
Why does dry HCl gas not change the colour of the dry litmus paper ?
Answer:
Dry HCl gas does not give H+ ions
and therefore does not change the colour of dry litmus paper.
Question 4
While diluting an acid, why is it recommended that the acid should be added to
water and not water to the acid ?
Answer:
While diluting an acid it is recommended that the acid should be added to water
and not water to the acid because if water is added to concentrated acid to
dilute it, then a large amount of heat is evolved at once. This heat changes
some of the water to steam explosively which can splash the acid on one’s face
or clothes and cause acid burns.
Question 5
How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is
diluted ?
Answer:
When a given amount of an acid is added to water, there is a fixed number of
hydronium ions per volume of the solution. On dilution, the number of hydronium
ions per volume decreases and concentration decreases.
Question 6
How is the concentration of hydroxide ions (OH–) affected when excess base is dissolved
in a solution of sodium hydroxide ?
Answer:
The concentration of hydroxide ions will increase when excess base is dissolved
in a solution of sodium hydroxide, but it happens to a limited extent only
after which the concentration becomes almost constant.
Page Number: 28
Question 1
You have two solutions A and B. The pH of solution A is 6 and pH of solution B
is 8. Which solution has more hydrogen ion concentration? Which of this is
acidic and which one is basic ?
Answer:
A pH value of less than 7 indicates an acidic solution, while greater than 7
indicates a basic solution. Since solution A has more hydrogen ion
concentration, solution A is acidic and solution B is basic.
Question 2
What effect does the concentration of H+ (aq) ions have on the nature of
the solution ?
Answer:
More the concentration of H+ ions,
higher the acidic nature of the solution.
Question 3
Do basic solutions also have H+ (aq)
ions ? If yes, then why are these basic ?
Answer:
Basic solutions have H+ (aq)
ions. But these are far less in number than OH– ions that is responsible for their
basic nature.
Question 4
Under what soil condition do you think a farmer would treat the soil of his
fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or
chalk (calcium carbonate) ?
Answer:
If the soil is too acidic (having low pH) then it is treated with materials
like quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk
(calcium carbonate).
Page Number: 33
Question 1
What is the common name of the compound CaOCl2 ?
Answer:
Bleaching powder.
Question 2
Name the substance which on treatment with chlorine yields bleaching powder.
Answer:
Slaked lime Ca (OH)2.
Question 3
Name the sodium compound which is used for softening hard water.
Answer:
Sodium carbonate.
Question 4
What will happen if a solution of sodium hydrogen carbonate is heated. Give the
equation of the reaction involved ?
Answer:
Solution of sodium hydrogen carbonate on heating gives sodium carbonate and
carbon dioxide gas is evolved.
Question 5
Write an equation to show the reaction between plaster of Paris and water.
Answer:
NCERT Solutions for
Class 10 Science Chapter 2 Textbook Chapter End Questions
Question 1
A solution turns red litmus blue, its pH is likely to be
(a) 1
(b) 4
(c) 5
(d) 10
Answer:
(d) 10
Question 2
A solution reacts with crushed-egg shells to give a gas that turns lime water
milky. The solution contains
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
Answer:
(b) HCl
Question 3
10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a
given solution of HC1. If we take 20 mL of the same solution of NaOH, the
amount of HC1 solution (the same solution as before) required to neutralise it
will be
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Answer:
(d) 16 mL
Question 4
Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotic
(b) Analgesic
(c) Antacid
(d) Antiseptic
Answer:
(c) Antacid
Question 5
Write word equations and then balanced equations for the reaction taking place
when
(a) dilute sulphuric acid reacts with zinc granules
(b) dilute hydrochloric acid reacts with magnesium ribbon
(c) dilute sulphuric acid reacts with aluminium powder
(d) dilute hydrochloric acid reacts with iron filing
Answer:
(a) Zinc + dilute sulphuric acid → Zinc sulphate + Hydrogen
Zn (s) + H2SO4 (aq) → ZnSO4 (aq) + H2 (g)
(b) Magnesium ribbon + dil. Hydrochloric acid → Magnesium
chloride + Hydrogen
Mg (s) + 2 HCl (aq) → MgCl2 (aq)
+ H2 (g)
(c) Aluminium powder + dil. Sulphuric acid > Aluminium
sulphate + Hydrogen
2Al (s) + 3H2SO4 (aq) → Al2 (SO4)3 (aq) + 3H2 (g)
(d) Iron filings + Dilute hydrochloric acid > Ferric
chloride + Hydrogen
2Fe (s) + 6HCl (aq) → 2FeCl3 (aq)
+ 3H2 (g)
Question 6
Compounds such as alcohol and glucose also contain hydrogen but are not
categorised as acids. Describe an activity to prove it.
Answer:
Though compounds like alcohol and glucose contain hydrogen but they do not
ionise in the solution to produce H+ ions on passing current through
them.
(i) Take solutions of alcohols and glucose.
(ii) Fix two nails on a cork, and place the cork in 100 mL beaker.
(iii) Connect the nails to the two terminals of a 6 volt battery through a bulb
and a switch, as shown in the given Figure.
(iv) Now pour alcohol in the beaker and switch on the current.
(v) The bulb does not glow.
(vi) Repeat the experiment with glucose. The bulb does not glow in this case
also.
(vii) This means no ions or H+ ions are present in the solution.
This shows that alcohols and glucose are not acids.
Question 7
Why does distilled water not conduct electricity, whereas rainwater does ?
Answer:
Distilled water does not conduct electricity because it does not contain any
ionic compound (like acids, bases or salts) dissolved in it.
Rainwater, while falling to the earth through the atmosphere, dissolves an
acidic gas carbon dioxide from the air and forms carbonic acid (H2CO3). Carbonic acid provides hydrogen ions,
H+ (aq)
and carbonate ions, CO(aq)32to rainwater. Hence, due to the presence
of carbonic acid which provides ions to rainwater, the rainwater conducts
electricity.
Question 8
Why do acids not show acidic behaviour in the absence of water ?
Answer:
The acidic behaviour of acids is due to the presence of hydrogen ions, [H+ (aq) ions], in them. The acid
produces hydrogen ions only in the presence of water. So in the absence of
water, an acid will not form hydrogen ions and hence will not show its acidic
behaviour.
Question 9
Five solutions A, B, C, D and E when tested with universal indicator showed pH
as 4, 1, 11, 7 and 9 respectively. Which solution is
(a) Neutral
(b) Strongly alkaline
(c) Strongly acidic
(d) Weakly acidic
(e) Weakly alkaline
Arrange the pH in increasing order of hydrogen ion concentration.
Answer:
(a) D
(b) C
(c) B
(d) A
(e) E
Increasing order of hydrogen ion concentration
11 < 9 < 7 < 4 < 1
i. e., C < E < D < A < B
Question 10
Equal lengths of magnesium ribbons are taken in test tubes A and B.
Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test tube B. In which
test tube will the fizzing occur more vigorously and why ?
Answer:
Fizzing will occur more vigorously in test tube A. Hydrochloric acid (HCl) is a
strong acid whereas acetic acid (CH3COOH) is a weak acid. Being strong acid,
the hydrochloric acid solution contains a much greater amount of hydrogen ions
in it due to which the fizzing will occur more vigorously in test tube A
(containing hydrochloric acid). The fizzing is due to the evolution of hydrogen
gas which is formed by the action of acid on the magnesium metal of magnesium
ribbon.
Question 11
fresh milk has a pH of 6. How do you think the pH will change as it turns into
curd ? Explain your answer.
Answer:
pH of milk falls below 6 as it turns into curd due to the formation of lactic
acid during this process. Lactic acid present in it reduces its pH value.
Question 12
A milkman adds a very small amount of baking soda to fresh milk.
(a) Why does he shift the pH of the fresh milk from 6 to slightly alkaline ?
(b) Why does this milk take a long time to set as curd ?
Answer:
(a) Milk is made slightly alkaline so that it may not get sour easily due to
the formation of lactic acid in it.
(b) The alkaline milk takes a longer time to set into curd because the lactic
acid being formed has to first neutralise the alkali present in it.
Question 13
Plaster of Paris should be stored in a moisture proof container. Explain why?
Answer:
Plaster of Paris should be stored in a moisture proof container because the
presence of moisture can cause slow setting of plaster of Paris by bringing
about its hydration. This will make the plaster of Paris useless after sometime.
Question 14
What is a neutralisation reaction ? Give two examples.
Answer:
The reaction between an acid and a base to form salt and water is called a
neutralisation reaction.
Examples:
Question 15
Give two important uses of washing soda and baking soda.
Answer:
Uses of washing soda :
(i) Washing soda is used in glass, soap and paper industries.
(ii) It is used for removing permanent hardness of water.
Uses of baking soda :
(i) Baking soda is used as an antacid in medicines to remove acidity of the stomach.
(ii) Baking soda is used for making baking powder (used in making cakes, bread,
etc.).
NCERT Solutions for Class 10 Science (Chemistry) Chapter
2 Acids, bases, and salts are part of NCERT Solutions for Class 10
Science. Here we have given Class 10 Science NCERT Solutions Chapter 2.
Question 1
You have been provided with three test tubes. One of them contains distilled
water and the other two contain an acidic solution and a basic solution
respectively. If you are given only red litmus paper, how will you identify the
contents of each test tube?
Solution:
The contents of each test tube would be identified by change in colour of red
litmus paper. For example, when we wet the red litmus paper with the basic
solution, it changes into blue colour. Put the changed blue litmus paper in the
solution which turns the blue to red will be the acidic solution. The solution,
which has no effect on any litmus paper, will be neutral and hence it will be
distilled water.
Question 2
Why should curd and sour substances not be kept in brass and copper vessels?
Solution:
Curd and other sour foodstuffs contain acids, which can react with the metal of
the vessel to form poisonous metal compounds which can cause food poisoning and
damage our health.
Question 3
Which gas is usually liberated when an acid reacts with a metal?
Solution:
When an acid reacts with metal, a salt and hydrogen gas is formed. i.e
Question 4
Metal compound A reacts with dilute hydrochloric acid to produce effervescence.
The gas evolved extinguishes a burning candle. Write a balanced chemical
equation for the reaction if one of the compounds formed is calcium chloride.
Solution:
The gas that extinguishes a burning candle is carbon dioxide, which is formed
by the action of dilute hydrochloric acid on a metal carbonate and produces
effervescence. Now, since one of the compounds formed is calcium chloride, it
shows that the metal compound is calcium carbonate. Thus, the metal compound A
is calcium carbonate (CaCO3).
Calcium carbonate reacts with dilute hydrochloric acid to form calcium
chloride, carbon dioxide and water. This can be written as:
Question 5
Why do HCl, HNO3,
etc., show acidic characters in aqueous solutions while solutions of compounds
like alcohol and glucose do not show acidic character?
Solution:
An acid is a substance, which dissociates on dissolving in water to produce
hydrogen ions [H+(aq)
ions]. The acids like HCl, H2SO4, HNO3 and CH3COOH, etc., show acidic character
because they dissociate in aqueous solutions to produce hydrogen ions, H+(aq)
ions.
The compounds such as glucose and alcohol also contain hydrogen but they do not
show acidic character. The aqueous solutions of glucose and alcohol do not show
acidic character because the hydrogen in them does not separate out as hydrogen
ions [H+ (aq)
ions] on dissolving in water.
Question 6
Why does an aqueous solution of acid conduct electricity?
Solution:
The aqueous solution of an acid conducts electricity due to the presence of
charged particles called ions in it.
Question 7
Why does dry HCl gas not change the colour of the dry litmus paper?
Solution:
Dry HCl gas does not contain any hydrogen ions in it, so it does not show
acidic behaviour. In fact, dry HCl gas does not change the colour of dry litmus
paper as it has no hydrogen ions [H+(aq) ions] in it.
Question 8
While diluting an acid, why is it recommended that the acid should be added to
water and not water to the acid?
Solution:
Diluting an acid should be done by adding concentrated acid to water gradually
with stirring and not by adding water to concentrated acid.
The heat is evolved gradually when a concentrated acid is added to water for
diluting an acid and the large amount of water is easily absorbed.
If, however, water is added to concentrated acid to dilute it, a large amount
of heat is evolved at once. The heat generated may cause the mixture to splash
the acid on our face or clothes and cause acid burns.
Question 9
How is the concentration of hydronium ions (H3O+) affected when a solution of an acid
is diluted?
Solution:
When the concentrated solution of an acid is diluted by mixing water, the
concentration of hydronium ions H3O+ per unit volume decreases.
Question 10
How is the concentration of hydroxide ions (OH-) affected when excess base is
dissolved in water?
Solution:
When the solution of a base is diluted by mixing more water in it, the
concentration of hydroxide ions (OH– ions) per unit volume decreases.
Question 11
You have two solutions A and B. The pH of solution A is 6 and pH of solution B
is 8.
i. Which solution has more hydrogen ion concentration?
ii. Which of this is acidic and which one is basic?
Solution:
The pH of a solution is inversely proportional to its hydrogen ion
concentration. This means that the solution having lower pH will have more
hydrogen ion concentration. In this case, solution A(having a lower pH of 6)
will have more hydrogen ion concentration.Solution A is acidic and solution B
is basic.
Question 12
What effect does the concentration of H+(aq) ions have on the nature of the
solution?
Solution:
Acids produce hydrogen ions in water. So, when an acid is added to water, the
concentration of hydrogen ions in water increases. The solution of acid thus
formed will have more of hydrogen ions and it will be acidic in nature.
Question 13
Do basic solutions also have H+(aq)
ions? If yes, why are these basic?
Solution:
No, the basic solution doesn’t have H+ ions as the solution has excess of
hydroxide ions.
Question 14
Under what soil condition do you think a farmer would treat the soil of his
fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or
chalk (calcium carbonate)?
Solution:
Most often the soil in the fields is too acidic. If the soil is too acidic
(having low pH), it is treated with materials like quicklime (calcium oxide) or
slaked lime (calcium hydroxide) or chalk (calcium carbonate). Thus, a farmer
should add lime or slaked lime in his fields when the soil is too acidic.
Question 15
What is the common name of the compound CaOCl2?
Solution:
The common name of the compound CaOCl2 is bleaching powder.
Question 16
Name the substance that on treatment with chlorine yields bleaching powder.
Solution:
Calcium hydroxide is the substance that on treatment with chlorine yields
bleaching powder.
Question 17
Name the sodium compound, which is used, for softening hard water.
Solution:
Sodium carbonate (washing soda) is used for softening hard water.
Question 18
What will happen if a solution of sodium hydro carbonate is heated? Give the
equation of the reaction involved.
Sodium carbonate and carbon dioxide are evolved when sodium hydro carbonate is
heated.
Question 19
Write an equation to show the reaction between plaster of Paris and water.
Solution:
Plaster of Paris has a very remarkable property of setting into a hard mass on
wetting with water. So, when water is added to plaster of Paris, it sets into a
hard mass in about half an hour. The setting of plaster of Paris is due to the
hydration crystals of gypsum, which set to form a hard, solid mass.
Question 20
Why does distilled water not conduct electricity, whereas rainwater does?
Solution:
Distilled water does not conduct electricity because it does not contain any
ionic compound (like acids, bases or salts) dissolved in it. On the other hand,
rain water conducts electricity. This can be explained as follows: Rain water,
while falling to the earth through the atmosphere, dissolves an acidic gas
carbon dioxide from the air and forms carbonic acid (H2CO3). Carbonic acid provides hydrogen ions,
H+(aq),
and carbonate ions, CO2-3 (aq), to rain
water. So, due to the presence of carbonic acid (which provides ions to rain
water), the rain water conducts electricity.
Question 21
Why do acids not show acidic behaviour in the absence of water?
Solution:
The acidic behaviour of acid is due to the presence of hydrogen ions. The acids
will not show its acidic behaviour in the absence of water, this is because the
acids produce hydrogen ions only in the presence of water.
Question 22
Five solutions A, B, C, D and E when tested with universal indicator showed pH
as 4, 1, 11, 7 and 9 respectively. Which solution is?
(i) Neutral?
(ii) Strongly alkaline?
(iii) Strongly acidic?
(iv) Weakly acidic?
(v) Weakly alkaline? Arrange the pH in increasing order of hydrogen-ion
concentration.
Solution:
Question 23
Equal lengths of magnesium ribbons are taken in test tubes A and B.
Hydrochloric acid (HCl) is added to test-tube A while acetic acid (CH3COOH) is added to test-tube B. In which
test-tube will fizzing occur more vigorously and why?
Solution:
Acetic acid (CH3COOH)
is a weak acid whereas hydrochloric acid (HCl) is a strong acid. Fizzing occurs
in the test tube due to the evolution of hydrogen gas by the action of acid on
magnesium ribbon. Since hydrochloric acid is a strong acid a large amount of
hydrogen gas is liberated in the test tube A. So fizzing occurs more vigorously
in test tube A .
Question 24
Fresh milk has a pH of 6. How do you think the pH will change as it turns into
curd? Explain.
Solution:
The pH will change to below 6, as lactic acid is formed when milk turns into
curd.
Question 25
Plaster of Paris should be stored in a moisture-proof container. Why?
Solution:
The presence of moisture can affect the slow setting of plaster of Paris by
bringing about its hydration. This will make the plaster of Paris useless after
some time.
Question 26
What is a neutralization reaction? Give two examples.
Solution:
The reaction of an acid and a base, giving rise to the corresponding salt and
water is called neutralization reaction.
Examples:
NaOH + HCl → NaCl + H2O
Mg(OH)2 +
H2CO3 → MgCO3 + 2H2O
Question 27
Give two important uses of washing soda and baking soda.
Solution:
Washing soda
1. It is often used as an electrolyte.
2. Domestically it is used as a water softener during laundry.
Baking soda
1. It is used to test garden soil for acidity. If it develops bubbles, the soil
is too acidic.
2. Washing a car with it can remove dead bug bodies without damaging the paint.
NCERT Solutions for Class 10 Science Chapter 2 (MCQs) [1 Mark
each]
Question 1.
Equal pieces of zinc granules are dropped in four test tubes. Following
substances are poured in all the four test tubes. The reaction will be vigorous
with [CCE 2014]
(a) CH3COOH
(b) HCl
(c) sodium bicarbonate solution
(d) lemon juice
Answer:
(b) Strong adds like HCl react vigorously with active metals like Zn and form
metal salt and evolve H2 gas.
Question 2.
Which of the following statements shows the property of an acid? [CCE 2014]
(a) It turns blue litmus to red
(b) It is sour in taste
(c) It has no effect on red litmus
(d) All of the above
Answer:
(d) An acid turns blue litmus red. Thus, it has no effect on red litmus and
acids are sour in taste.
Question 3.
A drop of a liquid sample was put on the pH paper. It was observed that the
colour of the pH paper turned blue. The liquid sample is [CCE 2014]
(a) lemon juice
(b) sodium bicarbonate solution
(c) distilled water
(d) hydrochloric acid
Answer:
(b) The liquid sample is of sodium bicarbonate (NaHC3) solution. It is a basic solution. And
we know that a basic solution turns pH paper blue.
Question 4.
Two solutions X and Y were found to have pH value of 4 and 10 respectively. The
inference that can be drawn is [CCE 2014]
(a) X is a base and Y is an acid
(b) Both X and Y are acidic solutions
(c) X is an acid and Yis a base
(d) Both X and Y are bases
Answer:
(c) Any solution having pH > 7 will be a base while the solution having pH
< 7 will surely be an acid. Hence, it can be concluded that X is an acid
(pH=4, i.e. < 7) and Yis a base (pH =10, i.e. > 7).
Question 5.
A student was asked to collect apparatus from lab store, for doing experiment
of pH of given sample. Identify the article which he is not supposed to pick.
[CCE 2014]
(a) pH paper
(b) Dropper
(c) Litmus paper
(d) Petri dish
Answer:
(d) Petri dish is not required for doing experiment of pH.
Question 6.
Which one of the following would you need to identify the gas that evolve when
you heat NaOH solution with zinc metal? [CCE 2014]
(a) Red litmus solution
(b) Blue litmus solution
(c) A burning splinter / matchstick
(d) Lime water
Answer:
(c) When a base like NaOH is treated with any active metal like Zn, it produces
H2 gas.
And the presence of the hydrogen gas can be tested by bringing a burning
splinter/ matchstick near the gas produced. The gas will burn with a pop sound
confirming the presence of hydrogen gas.
Question 7.
A solution has pH value of 5. On adding 10 mL of NaCl to it, what will be the
pH of the new solution?
(a) More than 5
(b) Less than 5
(c) Only seven
(d) No change in pH
Answer:
(d) As NaCl is a neutral solution due to its complete ionization in H2O and has pH = 7. Thus, it will not
affect the pH of the solution. Hence, the pH of the solution will remain
unaffected.
Question 8.
A salt is dissolved in water. The pH of this salt solution was found to be 7 by
measuring the pH with a universal indicator paper. The salt is most likely to
be
(a) Na2CO3
(b) KCl
(c) NH4Cl
(d) CH3COONa
Answer:
(b) Salts which are completely ionisable in water are said to be neutral salts
and their pH is equal to 7. Among the given salts, only KCl is completely
ionisable in the following manner: KCl (aq) → K+ + Cl
Hence, the pH of KCl will be 7.
Question 9.
A student performed an experiment using zinc granules and sodium carbonate with
sodium hydroxide and hydrochloric acid under different
conditions as shown below.
In which set up, no gas is evolved?
(a) I
(b) II
(c) III
(d) IV
Answer:
(d) Gas will not evolve in the case of IV set up as NaOH does not react with
sodium carbonate.
Question 10.
Four set ups as given below are arranged to identify the gas evolved when
dilute hydrochloric acid was added to zinc granules. Which is the most
appropriate set up?
(a) I
(b) II
(c) IV
(d) III
Answer:
(c) The gas evolved can be tested as shown in IV set up because to evolve H2 gas delivery tube, should not dip
in the acid.
Question 11.
Four students I, II, III and IV were asked to examine the changes for blue and
red litmus paper strips with dil. HCl (Solution A) and dil. NaOH (Solutions).
The following observations were reported by the 4 students. The sign ………….
indicates no colour change. [CCE 2015 ]
|
Litmus |
A |
B |
Litmus |
A |
B |
|
|
I |
Blue |
— |
red |
Blue |
red |
— |
|
II |
Red |
— |
blue |
Red |
— |
blue |
|
III |
Blue |
red |
red |
Blue |
blue |
blue |
|
IV |
Red |
blue |
blue |
Red |
red |
red |
The correct observation would be of student
(a) I
(b) II
(c) III
(d) IV
Answer:
(c) The correct observation is taken by student III because HCl (solution A) is
an acid which turns blue litmus red and dil. NaOH (solution B) is a base which
turns red litmus blue.
Question 12.
A sample of soil is mixed with water and allowed to settle. The clear
supernatant solution turns the pH paper yellowish orange. Which of the
following would change the colour of this pH paper to greenish blue? [NCERT
Exemplar]
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Answer:
(d) As pH paper turns greenish blue for weakly basic compound and antacids
contain weak base like Mg(OH)2.
So, an antacid would change the colour of this pH paper to greenish blue. Other
options (a) and (b) contain acids and option (c) is a neutral salt.
DR OMAR CLASSES BARRA-3, KANPUR
MOB-9450149685
Life Process
Introduction Life
Earth happens to be the only known planet
having life. There are beings which live, die and become part of nature again.
The living organism can be differentiated from the inanimate entities on
various parameters of life processes.
Life Process
Maintenance of living organism is essential
even if they are moving, resting or even sleeping. The processes which together
perform the function of maintenance of 'life' are called as life processes.
Nutrition, respiration, circulation, excretion are the examples of essential
life processes. In unicellular organisms, all these processes are carried out
by that single cell. In multicellular organisms, well-developed systems are
present to carry out the processes.
Nutrition
The process of acquiring food that
is needed for nourishment and sustenance of the organism is called nutrition.
There are two main modes of nutrition, autotrophic and heterotrophic.
Heterotrophic nutrition has subtypes as holozoic, saprophytic and parasitic
nutrition.
Autotrophic Nutrition
If an organism can
nourish itself by making its own food using sunlight or chemicals such
mode of nutrition is called as autotrophic nutrition. Plants photosynthesize
(use light energy) and are called photoautotrophs. Few bacteria use chemicals
to derive energy and are called chemoautotrophs.
Photosynthesis
Photosynthesis is the important
process by which food is formed. Life Processes The plants make food using
sunlight and water, which provides nourishment to other organism and
themselves. Chlorophyll present in the green parts absorbs light energy. This
light energy is used to split water into hydrogen and oxygen. Hydrogen is then
used to reduce carbon dioxide into carbohydrates, typically glucose.
Chlorophyll is essential for photosynthesis and stomata facilitate intake of
carbon dioxide.
DR OMAR CLASSES BARRA-3, KANPUR
MOB-9450149685
Stomata
Stomata are pores on the leaves that help in
exchange of gases. They are mostly found on the underside of the leaf. Each
stoma is guarded by guard cells, which control the opening and closing of the
pore. The water content of the guard cells is responsible for their function.
Stoma in open and closed state(Diagramm)
Saprophytic Nutrition
Some organism feed on dead and decaying
organic matter. This mode of nutrition is called saprophytic nutrition. The
food is partially digested outside the body and then it is absorbed. E.g.
Fungi are saprophytes.
Parasitic Nutrition
Some organisms feed on the expense
of another organism and in turn causing it harm. This is called parasitic mode
of nutrition. These organisms live on the body or in the body of a host
organism and derive the nutrients directly from the body of the host. E.g.
Leech is an ectoparasite while Ascaris is an endoparasite. Cuscuta is a
parasitic plant.
Nutrition in Amoeba
Amoeba feeds by holozoic mode of
nutrition. It engulfs the food particle using pseudopodia, the process is
called as phagocytosis. The engulfed food gets enclosed in a food vacuole. As
the food vacuole passes through the cytoplasm, digestion, absorption and
assimilation take place. When the food vacuole opens to outside, egestion of
undigested food takes place.
Holozoic Nutrition in Amoeba(Diagramm)
Nutrition in Paramoecium
Paramoecium also exhibit holozoic nutrition.
However, they have cilia that help them to engulf the food through the oral
groove. A food vacuole is created enclosing the food. It moves through the
cytoplasm, the process is called cyclosis. Food digested in the food vacuole is
absorbed by the cytoplasm. Undigested food is given out to a tiny
pore called anal pore or cytopyge.
Intracellular digestion in Paramecium(Diagramm)
DR OMAR CLASSES BARRA-3, KANPUR
MOB-9450149685
Nutrition in Humans
Humans are omnivores, they can eat
plant-based food as well as animal-based food. Being more complex, humans
have a very complicated nutrition system. The digestive system has an
alimentary canal and associated digestive glands, which together function to
nourish the body. There are five stages in human nutrition; Ingestion,
Digestion, Absorption, Assimilation and Egestion. Four stages i.e.
ingestion, digestion, absorption and egestion take place in the alimentary
canal while assimilation of food takes place in the whole body
Alimentary Canal
Alimentary canal in humans is a
long tube of varying diameter. It starts with mouth and ends with the
anus. Oesophagus, stomach, small intestine and large intestine are the parts of
the alimentary canal.
Mouth
It is the opening of the alimentary canal and
helps in ingestion of food. The buccal cavity which is present behind the mouth
is also commonly referred as mouth. The buccal cavity has teeth and
tongue. The set of teeth helps in mastication of food. The tongue has taste
buds on it and thus helps in tasting the food. The salivary glands open also in
the buccal cavity and pour saliva which initiates the process of digestion.
Teeth
Teeth are the hard structures present in the
buccal cavity. They help us to cut, shear and masticate the food we eat.
Vertical section of a tooth shows four layers as enamel, dentine, cement and
dental pulp. Enamel is outermost, shiny, highly mineralized and hardest part of
the human body. Dentine makes the bulk of the tooth and contains 70% inorganic
salts. Cement is present at the lining of a tooth and bony socket. Dental pulp
is the central soft part of a tooth and contains nerve endings, blood and lymph
vessels along with connective tissue. There are four types of teeth in humans,
Incisors, canines, molars and premolars, each with a specific function.
Incisors cut the food, canines tear the food while molars and premolars crush
it. The dental formula in adult humans is 2:1:2:3.
Structure of a Tooth(Diagramm)
DR OMAR CLASSES BARRA-3, KANPUR
MOB-9450149685
Oesophagus & Stomach
Oesophagus
The swallowed food passes into the oesophagus.
It is a muscular tube, about 25 cm long, with a sphincter (valve/opening) at
each end. Its function is to transport food and fluid, after being swallowed,
from the mouth to the stomach. Food is pushed down by peristaltic movements.
Stomach
The stomach is thick-walled
bag-like structure. Its receives food from the oesophagus at one end and opens
into the small intestine at the other end. The inner lining of the stomach
secretes mucus, hydrochloric acid and digestive juices. Food is churned into
semi-solid mass in the stomach and is called chyme. Enzymes present in the
gastric juice break down the food. Hydrochloric acid helps in partial
digestion of proteins and also kills harmful bacteria. Mucus secreted by the
wall of stomach resists the action of HCl on itself.
Small Intestine
The small intestine is the longest part of the alimentary
canal, about 20 feet long in humans. It has regions, duodenum, the region which
follows stomach, jejunum is the middle part and ileum is the later region
which continues further into the large intestine. The internal surface of the
small intestine is folded into finger-like projections called villi.
A common pancreatic duct from pancreas and liver opens into the duodenum.
Most of the chemical digestion and absorption takes place in the small
intestine.
Large Intestine
Large intestine in
humans is about 5 feet long. It has two regions, colon ( about 1.5 m) and
rectum (10 cm in length in the adult). The region of large intestine after
ileum is called colon while the last part is called rectum. Colon has three
regions as, ascending colon, transverse colon and descending colon. At the base
of ascending colon, a small finger-like out-growth is seen and is
called an appendix. It houses many useful bacteria required for digestion
of food. Rectum opens to outside by anus. The anus has internal and external
anal sphincters. Small and Large Intestine(Diagramm)
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
Peristalsis
A constant wave-like movement of the alimentary canal right
from the oesophagus to the small intestine is called as peristalsis. Muscles
present in the wall of the alimentary canal are responsible for peristalsis.
This movement helps to push the food through the alimentary canal.
Digestive Glands
Several glands produce digestive juices that help in
digestion of the food. Salivary glands, Gastric glands, Liver, Gallbladder,
Pancreas are few to name. Salivary glands secrete saliva which initiates
digestion in the mouth itself. Gastric glands present in the wall of the
stomach secrete hydrochloric acid and enzyme pepsin. The liver secretes bile
which is stored in the gallbladder. Bile helps in digestion of fats. The
pancreas secretes many digestive enzymes and its secretion is called as pancreatic
juice. Enzymes like trypsin, chymotrypsin, lipase, amylase are present in the
pancreatic juice.
Pancreas
The pancreas is a
long, flat gland present behind the stomach in humans. It is one of the major
digestive glands and is of mixed nature i.e. endocrine as well as exocrine. As
an endocrine organ, it secretes two hormones called insulin and glucagon which
maintain the blood sugar level. As an exocrine gland, it secretes pancreatic
juice which is nothing but a mixture of many digestive enzymes. The digestive
enzymes secreted by pancreas include trypsin and chymotrypsin and proteases
which digest proteins. It also includes amylase which digests the starch
content of the food. Pancreatic lipases are the pancreatic enzymes that help in
digestion of fats.
Anatomy of Human
Pancreas(Diagramm)
Holozoic Nutrition
The mode of nutrition
in which animals take their food as a whole is called as holozoic nutrition. In
holozoic nutrition, food passes through five steps as ingestion, digestion,
absorption, assimilation and egestion.
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
Physiology of Digestion
Mechanical digestion of food takes place in the buccal cavity
where teeth masticate the food, saliva gets mixed and it turns into a bolus.
Digestion of starch starts in the buccal cavity itself, with the action of
salivary amylase present in the saliva. Salivary amylase converts starch into
maltose. In the stomach, the churning of food takes place due to the muscular
contraction and relaxation of its wall. It breaks down the food into simpler
substances. Digestion of proteins starts in the stomach with the action of
pepsin. Proteins are broken down into smaller fragments called peptide by the
action of pepsin. The bolus after mixing with gastric juice, turn into a fine
soluble form known as the chyme. Chyme enters into the small intestine where
complete digestion takes place due to the action of various enzymes present in
the pancreatic juice, bile and intestinal juice. The digested food is
completely absorbed by the villi and microvilli of the small intestine.
Undigested food then enters into the large intestine. Colon is
responsible for absorption of water and salts whereas rectum stores the
undigested food temporarily before defaecation
Digestive System in Other Animals
Digestive systems in different animals vary in structure and
function. The structure of the digestive system depends on the food
habits of the animal. Alimentary canal in herbivores is long as the cellulose
content of their plant-based diet takes a long time to digest. On the other
hand, alimentary canal of carnivorous animals is comparatively shorter because
meat gets digested faster.
Anatomy of Digestive Tract
Alimentary canal in humans approximately 30 feet (9m) long.
It starts with mouth and ends in the anus. Between these two openings,
alimentary canal is the tube of varying diameter. Oesophagus, stomach, small
intestine (divided into three regions as duodenum, jejunum and ileum) and large
intestine(having two regions as colon and rectum) are the parts of the
alimentary canal. Salivary glands, pancreas and liver act as major digestive
glands. Glands present in the wall of the stomach and small intestine also
contribute towards digestion of food.
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
Role of HCl
Hydrochloric acid in
the stomach is secreted by the gastric glands present in its wall. pH of the
gastric acid is usually between 1.5 to 3.5 This acid serves
following functions: 1. Converts inactive pepsinogen and pro-rennin into active
pepsin and rennin respectively. 2. Provides acidic medium for protein
digestion. 3. Kills bacteria entered through food and prevents infection. 4.
Prevents putrefaction of food in the stomach. A thick layer of mucus secreted
by the mucus glands of the stomach prevent itself from the action of the
gastric acid. Excess acid damages gastric mucosa and causes gastric and
duodenal ulcers.
Salivary Glands
Salivary glands are the exocrine glands that secrete saliva
and through a system of ducts, it is poured into the mouth. In humans, three
major pairs of salivary glands are present, parotid, submandibular and
sublingual. In healthy individuals between 0.5 to 1.5 litres of saliva is
produced per day. Saliva serves following functions in the oral cavity. 1. It
lubricates and protects the soft and hard tissues of the oral cavity 2. It also
gives protection from dental caries 3. Saliva prevents microbial growth in the
oral cavity. 4. Saliva can encourage soft tissue repair by decreasing clotting
time and increasing wound contraction 5. Saliva contains the enzyme amylase
that hydrolyses starch into maltose and dextrin. Hence saliva allows digestion
to occur before the food reaches the stomach 6. Saliva acts as a solvent in
which solid particles can dissolve in and enter the taste buds located on the
tongue.
Salivary glands in Human(Diagramm)
Heterotrophic Nutrition
When an organism
depends on others for food, such a mode of nutrition is called as a
heterotrophic mode of nutrition. These organisms depend on autotrophs for their
nutritional requirements. E.g. Animals which eat plants as their food are
called herbivores. Animals which eat other animals as their food are called
carnivores. Holozoic, saprophytic and parasitic nutrition are all types of
heterotrophic nutrition.
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
Glandular Epithelium
Many small glands present in the inner layer of stomach and
intestine take part in the digestion of food. These glands are present in the
epithelial lining of stomach and intestine. The glands present in
different regions of the stomach are called as gastric glands. They are
responsible for the secretion of mucus, hydrochloric acid and enzymes like
pepsinogen. The glands present in the epithelial lining of the
small intestine and large intestine are called as intestinal glands.
Glands of the small intestine are responsible for the secretion of intestinal
juice also called as succus entericus. Intestinal juice
contains hormones, digestive enzymes, alkaline mucus, substances to
neutralize hydrochloric acid coming from the stomach. Intestinal
juice completes the digestion started by pancreatic juice. Glands of the large
intestine are associated with absorption of water and electrolytes
Villi and Micro Villi
Complete digestion and absorption of food take place in the
small intestine. Pancreatic juice coming from the pancreas, bile from the liver
and intestinal juice secreted by the intestinal glands complete the digestion
of food material. All the digested nutrients are absorbed by the long
finger-like projections present in the ileum of the small intestine. These
small finger-like projections of the inner wall of intestine are called as
villi (singular: villus). Each villus has its cell membrane of the lumen side
again folded into microscopic processes, called microvilli. Villi increase the
internal surface area of the intestinal walls making available a greater
surface area for absorption. Digested nutrients pass into the semipermeable
villi through diffusion. Villi also help in chemical digestion of food by
secreting digestive enzymes. Villi and microvilli of the small intestine
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
Liver
The liver is the largest and major digestive gland of humans
Liver, in humans, is located in the upper right-hand portion of the abdomen.
This organ is dark reddish brown in colour due to an extensive blood
supply. Some of the important functions of the liver are as follows: 1. It
secretes bile that helps in digestion. 2. It filters the blood coming from
digestive tract before passing it to the rest of the body. 3. It detoxifies
various metabolites and drugs 4. The liver makes proteins important for blood
clotting and other functions. 5. It stores and releases glucose as needed. 6.
It processes haemoglobin, from the dead and worn out RBCs, for the iron
content (the liver stores iron). 7. Conversion of harmful ammonia to urea takes
place in the liver.
Digestive Juices
Pancreatic juice, bile and intestinal juice (succus
entericus) are collectively called as digestive juices. A common duct from
digestive glands pours the secretions into the duodenum. Chyme enters into the
small intestine where complete digestion takes place due to the action of
various enzymes. In the duodenum, the acidity of chyme is turned to
alkalinity by the action of bile coming from the liver. This is necessary
for pancreatic enzyme action. Bile also emulsifies the fats into smaller
globules. Pancreatic and intestinal amylases break down the carbohydrates into
glucose. Trypsin and chymotrypsin are the proteases responsible for the
breakdown of proteins finally into amino acids. Lipase is the enzyme which acts
on the emulsified fats and breaks them down into glycerol and fatty acids.
Water Absorption in Large Intestine
The large intestine is
not involved in digestion of food or absorption of nutrients. The major
function of the large intestine is to absorb water from the remaining
indigestible food matter and make the stool solid. The large intestine also
helps in absorption of vitamins made by bacteria that normally live in the
large intestine. The innermost layer of the large intestine also acts as a
barrier and protects from microbial infections and invasions. Rectum stores the
undigested food temporarily until defaecation.
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
Respiration
Introduction to
Respiration
Respiration broadly
means the exchange of gases. Animals and plants have different means of
exchange of gases. At a cellular level, respiration means the burning of the
food at the for generating the energy needed for other life processes. Cellular
respiration may take place in the presence or absence of oxygen.
A General Reaction of Respiration(Diagramm)
Respiration in Humans
The human respiratory system is more complex and involves
breathing, exchange of gases and cellular respiration. A well defined
respiratory system helps breathing and exchange of gases. Breathing involves
inhalation of oxygen and exhalation of carbon dioxide. The gaseous exchange
takes place in the lungs and oxygen is supplied to all cells of the body.
Cellular respiration takes place in each and every cell.
Respiratory System
The human respiratory system involves the nose, nasal
cavities, pharynx, larynx, trachea/windpipe, bronchi, bronchioles and alveoli.
Bronchioles and alveoli are enclosed in a pair of lungs. Rib cage, muscles
associated with the rib cage and diaphragm, all help in inhalation and
exhalation of gases. Exchange of gases takes place between an alveolar surface
and surrounding blood vessels. Alveoli provide a large surface area for
exchange of gases.
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
Physiology of Respiration
Breathing in humans is
facilitated by the action of internal intercostal and external intercostal
muscles attached to the ribs and the diaphragm. When the dome-shaped diaphragm
contracts and becomes flattened and the rib cage is expanded due to the action
of intercostal muscles, the volume of the lungs increases, pressure there drops
down and the air from outside gushes in. This is inhalation. To exhale, the
diaphragm relaxes, becomes dome-shaped again, chest cavity contracts due to the
action of intercostal muscles, the volume inside the lungs decreases, pressure
increases and the air is forced out of the lungs. Inhaled air increases the
concentration of oxygen in the alveoli, so oxygen simply diffuses into
the surrounding blood vessels. Blood coming from cells has more concentration
of carbon dioxide than outside air and thus carbon dioxide simply diffuses
out of the blood vessels into the alveoli. Thus, breathing takes place due to
the combined action of intercostal muscles and diaphragm while the exchange of
gases takes place due to simple diffusion.
Breathing Mechanism in
Human(Diagramm)
Inhalation and Exhalation
The process of taking
in air rich in oxygen is called inhalation. Similarly, the process of
giving out air rich in carbon dioxide is called exhalation. One breath
comprises one inhalation and one exhalation. A person breathes several times in
a day. The number of times a person breathes in one minute is termed as
his/her breathing rate.
Diffusion
Diffusion is
the movement of molecules from high concentration area to the low
concentration area without spending any enrgy.
Diffusion of gas molecules(Diagramm)
Cellular Respiration
Cellular respiration
is set of metabolic reactions occurring inside the cells to convert biochemical
energy obtained from the food into a chemical compound called adenosine
triphosphate (ATP). Metabolism refers to a set of chemical reactions
carried out for maintaining the living state of the cells in an organism.
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
These can be divided into two categories: Catabolism –
the process of breaking molecules to obtain energy. Anabolism – the
process of synthesizing all compounds required by the cells. Therefore,
respiration is a catabolic process, which breaks large molecules into smaller
ones, releasing energy to fuel the cellular activities. Glycolysis, Krebs cycle
and electron transport chain are the important processes of the cellular
respiration.
Interlinking of Breathing and Cellular Respiration(Diagramm)
Aerobic Respiration
Aerobic respiration is a process in which the food i.e.
glucose is converted into energy in the presence of oxygen. The general
equation of aerobic respiration as a whole is as given below
Glucose + oxygen ⇒Carbon-dioxide +
Water + Energy
This type of respiration takes place in animals, plants and
other living organisms.
Respiration in Lower Animals
Lower animals lack a sophisticated respiratory system like
lungs, alveoli etc. Respiration in them takes place by simple
exchange mechanisms. Animals like earthworms take in gases through their skin.
Fishes have gills for gaseous exchange. Insects have a tracheal system, which
is a network of tubes, through which air circulates and gaseous exchange takes
place. Frogs breathe through their skin when in water and through their lungs
when on land.
Respiration in Muscles
Respiration in muscles can be anaerobic when there is not
enough oxygen. Glucose gets broken down to carbon dioxide and lactic acid.
This results in the accumulation of lactic acid that makes the muscles sore.
This type of anaerobic respiration is also known as lactic acid fermentation
ATP
It is the energy
currency of the cell. ATP stands for Adenosine Tri-Phosphate. This molecule is
created as a result reaction like photosynthesis, respiration etc. The three
phosphate bonds present in the molecule are high energy bonds and when they are
broken, a large amount of energy is released. Such released energy is then used
for other metabolic reactions. Structure of ATP(Diagramm)
DR OMAR CLASSES BARRA 3, KANPUR MOBILE 9450149685
Respiration in Plants
Unlike animals and humans, plants do not have any specialized
structures for gaseous exchange They have stomata (present in leaves) and
lenticels (present in stems) which are involved in the exchange of gases.
Compared to animals, plant roots, stems, and leaves respire at a very lower
rate.
Transpiration
Transpiration is a biological process in which water is lost
in the form of water vapour from the aerial parts of the plants. This
process occurs mainly through the stomata where the exchange of gases (oxygen
and carbon dioxide) occurs. Transpiration helps in transportation of
water from roots to upper parts of plants and this is explained by
'transpirational pull theory'. Loss of water, especially from leaves, acts as
straw effect and pulls water upwards from roots. Transpiration also acts as an
excretoy mechanism in plants as it helps to get rid of excess water
Why Do We Need Lungs
In unicellular organisms like an amoeba exchange of gases
takes place through a general body surface by osmosis. In lower animals like an
earthworm, the gaseous exchange takes place through their moist skin. The
requirement of oxygen is sufficiently met by these ways. But as the animal
starts becoming more and more complex, for example, human, the requirement of
oxygen cannot be met alone by diffusion. Moreover, diffusion will not be able
to supply oxygen to the deep-seated cells. This difficulty has led to the
evolution of a more complex mechanism of gaseous exchange and that is the
development of lungs. The alveoli present in the lungs provide large surface
area required for the necessary gas exchange.
Transportation in Human Beings
Transportation
All living
organisms need few necessary components like air, water, and food for
their survival. On our regular basis, animals ensure these elements by
breathing, drinking and eating. The required elements are transported to
their body cells and tissues by a transportation system. In plants,
vascular tissue is responsible for transporting the substances.
DR OMAR CLASSES BARRA 3, KANPUR MOBILE-9450149685
DR OMAR CLASSES BARRA 3, KANPUR MOBILE-9450149685
Transportation
in Humans
Transportation
in humans is done by the circulatory system. The circulatory system in humans
mainly consists of blood, blood vessels and heart. It is responsible for the
supply of oxygen, nutrients, removal of carbon dioxide and other excretory
products. It also helps to fight the infections.
Heart
The
muscular organ which is located near the chest slightly towards the left in
the thoracic region. The heart is the main pumping organ of the body. The
human heart is divided into four chambers which are involved in the
transportation of oxygenated and deoxygenated blood. The upper two chambers are
called as atria whereas the lower two chambers are called
as ventricles. Human Heart(Diagramm)
Blood
Vessels
Blood
vessels carry blood throughout the body. There three types of blood vessels;
arteries, veins and blood capillaries. Arteries carry oxygenated blood and
veins carry deoxygenated blood. Gaseous exchange takes place between blood and
cells at capillaries.
Difference between Arteries and Veins
Arteries
Veins
1 An
artery carries blood away from heart
A vein carries blood towards the heart
2 An artery
is thicker than a vein A
vein is comparitively thinner
3 The
Pulmonary artery carries The
pulmonary vein carries deoxygenated blood oxygenated blood
4
Arteries have rigid walls Veins
have comparitively thinner walls
5 Blood
flows under pressure through an artery Blood
flow through vein is much calmer
6 Lumen
of arteries is narrow Lumen
of veins is comparitively wider
DR OMAR
CLASSES BARRA 3, 9450149685
Class 10 Science Notes Chapter
7 Control and Coordination:
.The Nervous System
Movement in
organisms
The ability of organisms to move certain body parts is movement.
When
they move from one place to another, it is called locomotion.
Organisms show movements in response to stimuli.
Introduction to control
& coordination
- Organisms
move in response to various kinds of stimuli like light, heat,
nutrients/food, etc.
- All
the activities in animals are controlled and coordinated by the nervous
and endocrine system.
- Hormones
are chemical messengers, which assist the nervous system in carrying out
various functions. They are secreted by endocrine glands.
- Hormones
in plants coordinate the movements.
THE NERVOUS SYSTEM
Neuron
Neuron
is the structural and functional unit of the nervous system.
- Each
neuron has three main parts: dendrites, cyton/soma/cell body and
axon.
- Dendrites receive
impulses from other neurons.
- Cyton/soma
processes the impulse.
- Axon
transmits the impulse, either to another neuron or to muscles/glands, etc.
- Axon
may be myelinated or non-myelinated.
- The impulse transmission is faster in myelinated neurons.
DR OMAR
CLASSES BARRA 3, 9450149685
Central nervous system
The central nervous system (CNS) is made up of the brain and the
spinal cord. Functions of different parts of the brain are:
- Cerebrum
is responsible for reasoning, logic, emotions, speech, memory, visual
processing, recognition of auditory and taste stimuli, etc.
- Cerebellum
regulates and coordinates body movements, posture and balance.
- Pons
relays signals from hindbrain to forebrain.
- Medulla
Oblongata controls all involuntary movements like vomiting, sneezing,
yawning, heartbeat, breathing, blood pressure, etc.
- Medulla
oblongata continues as the spinal cord which runs through the vertebral
column and it controls reflex actions.
Peripheral nervous system
- The
nerves given out by the brain and the spinal cord form the peripheral
nervous system (PNS).
- There
are 12 cranial nerves and 31 spinal nerves in humans.
Somatic nervous system
- It
forms a part of the PNS.
- The
nerves of PNS that control the voluntary actions of the body form the
somatic nervous system.
Autonomic nervous system
- All
the nerves of the PNS that control the involuntary actions in the body
form the autonomic nervous system.
DR OMAR
CLASSES BARRA 3, MOB 9450149685
- Two
divisions of autonomic nervous system are: sympathetic and
parasympathetic nervous system.
- The sympathetic
nervous system prepares the body for intense physical activity and is
often referred to as the fight-or-flight response, while
the parasympathetic nervous system has almost the exact opposite
effect and relaxes the body and inhibits or slows many high energy
functions.
Reflex action
Reflex action is a sudden, involuntary
reaction of the body in response to stimuli.
Reflex arc
- It
is the path followed by electrical impulse during a reflex
action.
- The
impulse travels from the receptor organ to the spinal cord/brain. It is
processed there and the information is brought back to the concerned
muscle to carry out the action.
- Thus,
receptor organ, sensory/afferent neuron, interneuron, motor/efferent
neuron and effector organ are the components of a reflex arc.
Protection of CNS
The brain is protected by 3 main layers –
- The
bony skull (cranium)
- The
cerebrospinal fluid
- The
meninges (Dura mater, Arachnoid and Pia mater).
Plant Hormones and Movements
Plant hormones
Control and coordination in plants are carried out by
hormones.
|
Plant Hormone |
Function |
|
Auxin |
Helps
in Growth of Plant Tissue |
|
Cytokinin |
Promotes
Cell division, delays aging of cells |
|
Gibberilins |
Helps
in growth of stems, initiates seed germination, promotes flowering, cell
division and seed growth after germination |
|
Abscisic
acid |
Inhibits
growth and causes wilting of leaves, promotes dormancy of buds and seeds |
|
Ethylene |
This
is a gaseous hormone which causes ripening of fruits |
Growth independent movements
The
movements which are not growth related are called nastic movements. These
movements occur in response to environmental stimuli but the
direction of response is not dependent on the direction of the stimulus.
- The
movement in the touch-me-not plant is thigmonastic movement (movement
in response to touch).
Growth-related movements in plants
The
movements which are growth related are called tropic movements. These
movements occur in response to environmental stimuli and the direction of
the response is dependent on the direction of the stimulus.
Examples:
- Phototropic movement (light dependent)
- Geotropic movement (gravity dependent)
- Chemotropic movement (chemical
dependent)
- Hydrotropic movement (water dependent)
- Thigmotropic movement (touch dependent)
Geotropism
Movement
of plant parts in response to earth’s gravitational force is known as
geotropism/gravitropism.
- Towards gravity – positive geotropism
- Away from gravity – negative geotropism
- Root grows towards gravity and shoot
grows away from gravity
Phototropism
Movement
of plant parts in response to light is known as phototropism.
- Towards light – positive phototropism
- Away from light – negative phototropism
- Stems move towards light and roots
move away from light
\
Hydrotropism
Movement
of plant parts in response to water or moisture.
- Towards water – positive hydrotropism
- Away from water – negative hydrotropism
- Again, root movement in search of water
is positive hydrotropism
Chemotropism
Movement
of plant parts in response to chemical stimuli is known as chemotropism.
- Towards chemical – positive chemotropism
- Away from chemical – negative
chemotropism
- The growth of pollen tube towards the
ovule is positive chemotropism
Thigmotropism
Movement
of plant parts in response to touch is called as thigmotropism.
- Towards touch – Positive thigmotropism
- Away from touch – negative thigmotropism
- Movement of tendrils around the support
is positive thigmotropism
The Endocrine System
Exocrine glands
Exocrine
glands are glands that discharge secretions by means of ducts, which
open onto an epithelial surface.
Endocrine glands
Endocrine
glands are the ductless glands which secrete hormones into the bloodstream in
humans.
Pituitary gland
- It is a pea-sized gland located at the
base of the brain.
- It is the master gland as it controls
the secretions of all the other endocrine glands.
- It also secretes Growth Hormone (GH).
Under-secretion of GH causes ‘Dwarfism’ and over-secretion causes
‘Gigantism’ in children and ‘Acromegaly’ in adults.
Thyroid gland
- It is a butterfly-shaped gland located
in the throat.
- It secretes the hormone ‘Thyroxine’
which regulates the metabolism of the body.
- Iodine is required to synthesize
thyroxine in the body.
- In case of iodine deficiency,
under-secretion of thyroxine leads to goitre.
Pancreas
- It is a leaf-like gland present behind
the stomach in the abdomen.
- It is an endocrine as well an exocrine
gland.
- As an endocrine gland, it manufactures
two hormones – Insulin and glucagon. Both these hormones act
antagonistically and regulate the sugar level in the blood.
- As an exocrine gland, it
secretes enzymes to break down the proteins, lipids, carbohydrates
and nucleic acids in food.
- Insufficient amount of insulin from
pancreas leads to diabetes.
Adrenal gland
- Occurs in pair above each kidney.
- It decreases in size with age.
- Secrets the hormone adrenaline which
helps in flight and fight response.
- Also secretes nor adrenaline
Gonads
- Gonads are the gamete-producing organs –
testes in males and ovaries in females.
- The testes produce the male hormone
testosterone and ovaries produce the female hormones oestrogen and
progesterone.
- Testosterone and oestrogen help in
producing gametes and are responsible for the sexual characteristics
in males and females respectively.
- Progesterone is the pregnancy hormone..
Other endocrine
organs
- The other endocrine organs include the
hypothalamus, parathyroid, pineal and thymus glands.
Frequently asked
Questions on CBSE Class 10 Science Notes Chapter 7: Control and Coordination
What
is the function of the Central nervous system?
The central nervous system
(CNS) controls most functions of the body and mind. It consists of two parts:
the brain and the spinal cord. The brain is the center of our thoughts, the
interpreter of our external environment, and the origin of control over body
movement.
What
are some facts about the human brain?
1. 60% of the human brain is
composed of fat 2. Brain contains about 100 billion neurons and 100 trillion
connection 3. The texture of brain is siilar to that of firm jelly
How
many parts does the human eye have?
The human eye totally consists
of 7 parts that work together.


