Matter in Our Surroundings Class 9 Notes Science Chapter 1
Matter in Our Surroundings Class 9 Notes – Here We have provided summary and revision notes for Class 9 Science Chapter 1. This CBSE notes contains CBSE Key Notes, CBSE Revision Notes, Short Key Notes, images, diagrams of the complete Chapter 1 titled Matter in Our Surroundings of Science taught in class 9. If you are a student of class 9 who is using NCERT Textbook to study Science, then you must come across Chapter 1 Matter in Our Surroundings. After you have studied lesson, you must be looking for notes to memorize. Here you can get complete Chapter 1 Matter in Our Surroundings class 9 notes in one place. For a better understanding of this chapter, you should also see NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings.
CBSE Class 9 Science Notes Chapter 1 Matter in Our Surroundings Pdf free download is part of Class 9 Science Notes for Quick Revision. Here we have given NCERT Class 9 Science Notes Chapter 1 Matter in Our Surroundings.
CBSE Class 9 Science Notes Chapter 1 Matter in Our Surroundings
Facts that Matter
Introduction
- Everything in this universe is made of materials which scientist has names ‘matter’.
- The matter is made up of very small tiny particles. It is not continuous but is particulate.
- The matter is anything that occupies space and has mass.
- Particles of matter have space between them and are continuously moving.
- Particles of matter attract each other.
States of Matter: It has 3 states.
Matter can change its state from solid to liquid and from liquid to gas and vice-versa.
Effect of temperature: On increasing the heat, the particles gain energy and start vibrating with greater energy. Due to increased kinetic energy the particles overcome the force of attraction and a new state is obtained.
Melting point: The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
Boiling point: The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. Boiling is a bulk phenomenon.
Latent heat of fusion: The amount of heat energy required to change 1 kg of a solid into liquid at its melting point is called the latent heat of fusion of the solid.
Latent heat of vaporization: The amount of heat energy required to change 1 kg of a liquid to vapour at atmospheric pressure, at its boiling point is called the latent heat of vaporization of the liquid.
Effect of change of pressure on the matter: On applying pressure, the particles of matter can be brought close together and the state of matter can be changed. For example, CO2 gas can be solidified by applying pressure and lowering temperature.
Evaporation: The phenomenon of changing of a liquid into its vapour state at any temperature below its boiling point is called evaporation. Evaporation is a surface phenomenon.
Factors affecting evaporation.
- An increase in surface area increases evaporation.
- An increase in temperature increases the rate of evaporation.
- A decrease in humidity increases the rate of evaporation.
- An increase in wind speed increases the rate of evaporation.
- Evaporation causes a cooling effect.
Some measurable quantities and their units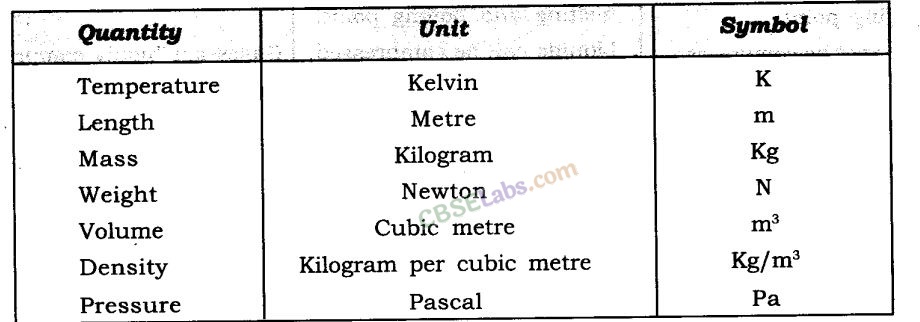
No comments:
Post a Comment