DR OMAR CLASSES
BARRA 3, MOBILE 9450149685
Introduction to
Structure of an Atom
Atoms
Atoms
are the building blocks of matter. It is the smallest unit of matter that is
composed of three sub-atomic particles: the proton, the neutron and the
electron.
Cathode ray
experiment
- J.
J. Thomson discovered the existence of electrons.
- He
did this using a cathode
ray tube, which is a vacuum-sealed tube with a cathode and
anode on one end that created a beam of electrons travelling towards the
other end of the tube.
- The
air inside the chamber is subjected to high voltage and electricity flows
through the air from the negative electrode to the positive electrode.
- The
characteristics of cathode rays (electrons) do not depend upon the
material of electrodes and the nature of the gas present in the cathode
ray tube.
- The experiment showed that the atom was not a simple, indivisible particle and contained at least one subatomic particle – the electron.
Apparatus of the experiment
Electrons
- Electrons
are the negatively charged sub-atomic particles of an atom.
- The
mass of an electron is considered to be negligible, and its charge is -1.
- The
symbol for an electron is e–
- Electrons
are extremely small.
- They are
found outside the nucleus.
DR OMAR CLASSES
BARRA 3, MOBILE 9450149685
Thomson’s model of
an atom
- According
to Thomson,(i) An atom consists of a positively charged sphere and the
electrons are embedded in it. (ii) The negative and positive charges are
equal in magnitude. So, the atom as a whole is electrically neutral
- The
first model of an atom to be put forward and taken into consideration.
- He
proposed a model of the atom be similar to that of a Christmas
pudding/watermelon.
- The
red edible part of the watermelon is compared with the positive charge in
the atom.
- The
black seeds in the watermelon are compared with the electrons which are
embedded on it.
Radioactivity
Radioactivity
- Radioactivity
is the term for the process by which an unstable nucleus of an atom loses
energy by giving out particles.
- It
does so by giving out particles such as alpha and beta particles.
- This
process is spontaneous.
- An
atom is unstable if the nucleus has an imbalance, meaning a difference in
the protons and neutrons.
Rutherford Model
Rutherford’s
experiment and observations
In this experiment, fast-moving alpha (α)-particles were made to
fall on a thin gold foil. His observations were:
DR OMAR CLASSES
BARRA 3, MOBILE 9450149685
- A
major fraction of the α-particles bombarded towards the gold sheet passed
through it without any deflection, and hence most of the space in an atom is
empty.
- Some
of the α-particles were deflected by the gold sheet by very small angles,
and hence the positive
charge in an atom is not uniformly distributed.
- The
positive charge in an atom is concentrated in a very
small volume.
- Very
few of the α-particles were deflected back, that is only a few
α-particles had nearly 180o angle of deflection. So the volume occupied by the positively
charged particles in an atom is very small as compared to the total volume
of an atom.
Rutherford’s model
of an atom
Rutherford concluded the model of the atom from the α-particle
scattering experiment as:
(i) There is a positively charged centre in an atom called the
nucleus. Nearly all the mass of an atom resides in the nucleus.
(ii) The electrons revolve around the nucleus in well-defined
orbits.
(iii) The size of the nucleus is very small as compared to the
size of the atom.
Rutherford’s
Model
Drawbacks of
Rutherford’s model
- He
explained that the electrons in an atom revolve around the nucleus in
well-defined orbits. Particles in a circular orbit would experience
acceleration.
- Thus,
the revolving electron would lose energy and finally fall into the
nucleus.
- But
this cannot take place as the atom would be unstable and matter would not
exist in the form we know.
DR OMAR CLASSES
BARRA 3, MOBILE 9450149685
Be More Curious!!!
- The
Millikan’s Oil Drop Experiment was an experiment performed by Robert A.
Millikan and Harvey Fletcher in 1909 to measure the charge
of an electron.
- In
the experiment, Millikan allowed charged tiny oil droplets to pass through
a hole into an electric field.
- By
varying the strength of electric field, the charge over an oil droplet was
calculated, which always came as an integral value of ‘e.’
- The
conclusion of this is that the charge is said to be quantized, i.e. the
charge on any particle will always be an integral multiple of e which is
1.6*10-19
Neil Bohr Model
Properties of
electrons, protons and neutrons
Bohr’s Model of an atom
Bohr came up with these postulates to overcome the objections
raised against Rutherford’s model:
- Electrons
revolve around the nucleus in stable orbits without emission of radiant
energy. Each orbit has a definite energy and is called an energy shell or
energy level.
- An
orbit or energy level is designated as K, L, M, N shells. When the
electron is in the lowest energy level, it is said to be in the ground
state.
- An
electron emits or absorbs energy when it jumps from one orbit or energy
level to another.
- When it jumps from a higher energy level to lower energy level, it emits energy while it absorbs energy when it jumps from lower energy level to higher energy level.
Bohr’s Model
Orbits
Orbits are energy shells surrounding the nucleus in which
electrons revolve.
Electron
distribution in different orbits
The distribution was suggested by Bohr and Bury;
- The
maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is
the orbit number or energy level index, 1,2,3,….
- The
maximum number of electrons in different shells are as follows: the first
orbit will have 2*12=2, the second orbit will have
2*2Msup>2=8, the third orbit will have 2*32=18, fourth
orbit 2*42=32 and so on.
- The
shells are always filled in a step-wise manner from the lower to higher
energy levels. Electrons are not filled in the next shell unless previous
shells are filled.
Valency
- The
electrons present in the outermost shell of an atom are known as the
valence electrons.
- The
combining capacity of the atoms or their tendency to react and form
molecules with atoms of the same or different elements is known
as valency of the atom.
- Atoms
of elements, having a completely filled outermost shell show little
chemical activity.
- Their
combining capacity or valency is zero.
- For
example, we know that the number of electrons in the outermost shell of
hydrogen is 1, and in magnesium, it is 2.
- Therefore,
the valency of hydrogen is 1 as it can easily lose 1 electron and become
stable.
- On
the other hand, that of magnesium is 2 as it can lose 2 electrons
easily and also attain stability.
Atomic Number
The number of protons found in the nucleus of an atom is termed
as the atomic number. It is denoted by the letter ‘Z’.
Mass number and
representation of an atom
Protons and neutrons are present in the nucleus, so the mass
number is the total of these protons and neutrons.
DR OMAR CLASSES
BARRA 3, MOBILE 9450149685
Isotopes and
Isobars
Isotopes are defined as the atoms of the same element, having
the same atomic number ( number of protons ) but different mass numbers (
number of protons+neutrons ).
For example: In the case of Hydrogen we have:
Atoms of different elements with different atomic numbers, which have the same
mass number, are known as isobars.
For example, Calcium and Argon: both have the same mass number – 40
20Ca40 and 18Ar40
Calculation of
mass number for isotopic elements
When an element has an isotope, the mass number can be
calculated by the different proportions it exists in.
For example take 98% Carbon-12u and 2% Carbon-13u
This does not mean that any Carbon atoms exists with the mass
number of 12.02u. If you take a certain amount of Carbon, it will contain both
isotopes of Carbon, and the average mass is 12.02 u.
Chapter 4:
Structure of an Atom
What are the
features of ‘Thomas atomic model’?
J.J. Thomson’s experiments with cathode ray tubes showed that
all atoms contain tiny negatively charged subatomic particles or electrons.
Thomson proposed the plum pudding model of the atom.
Who was ‘Ernest Rutherford’?
Ernest Rutherford was a New Zealand physicist who came to be
known as the father of nuclear physics.
What did ‘Neils
Bohr’ discover?
Niels Bohr proposed a theory for the hydrogen atom, based on
quantum theory that some physical quantities only take discrete values.
DR OMAR CLASSES BARRA MOB-9450149685
CBSE
Class 9 Science Notes Chapter 5 The Fundamental Unit of Life
Facts that Matter
The
smallest functional unit of life is a cell, discovered by Robert Hooke in 1665.
A cell can independently perform all necessary activities to sustain life.
Hence cell is the basic unit of life.
There are two types of cells plant cell and animal cell. The different cell
organelles and their functions are as follows:
1.
Plasma/Cell membrane: This
is the outermost covering of the cell that separates the contents of the cell
from its external environment. The plasma membrane allows or permits the entry
and exit of some materials in and out of the cell so the cell membrane is
called a selectively permeable membrane.
Some
substances like CO2 or O2 gases can move
across the cell membrane by a process called diffusion. The movement of water
molecules (liquid) through such a selectively permeable membrane is called
osmosis. Osmosis is the passage of water from a region of high water
concentration through a semi-permeable membrane to a region of low water concentration.
If the medium surrounding the cell has a higher
water concentration than the cell, the cell will gain water by osmosis. Such a
solution is known as a hypotonic solution.
If the medium has exactly the same water concentration as the cell, there will be no net movement of water across the cell membrane. Such a solution is known as an isotonic solution. If the medium has a lower water concentration then the cell will lose water by osmosis. Such a solution is known as a hypertonic solution.
The plasma membrane is flexible and is made up of organic molecules called lipids and proteins. The flexibility of cell membrane also enables the cell to engulf in food and other material from its external environment. Such process is known as endocytosis. It is observed in Amoeba.
2. Cell wall (Protective
wall): Plants
cells, in addition to the plasma membrane have another rigid outer covering
called cell wall. The cell wall lies outside the plasma membrane. The plant
cell wall is mainly composed of cellulose. It is a complex substance and
provides structural strength to plant cells. When a living plant loses water
through osmosis there is shrinkage or contraction of contents of the cell away
from cell wall. This phenomenon is known as plasmolysis.
3. Nucleus (Brain of a cell): The nucleus has a
double-layered covering called nuclear membrane. The nuclear membrane has pores
which allow the transfer of material from inside the nucleus to its outside,
i.e., to the cytoplasm.
DR OMAR CLASSES BARRA MOB-9450149685
The nucleus contains chromosomes, which are visible as rod-shaped structures only when the cell is about to divide.
Chromosomes — contain information for inheritance of features from parents to next generation in form of DNA [Deoxyribo Nucleic Acid] molecules. Chromosomes are composed of DNA and protein. Functional segments of DNA are called genes. The nucleus plays a central role in cellular reproduction.
Prokaryotic Cells: In some organisms
like bacteria, the nuclear material is not enclosed by nuclear membrane and
membrane bound cell organelle are absent. Such nucleus is called nucleoid and
such cells are known as prokaryotic cell. Such cells have single chromosome.
Eukaryotic Cells: Cells having well defined nucleus and having membrane bound
cell organelle is termed as eukaryotic cell. Such cells have more than one
chromosomes.
4. Cytoplasm: The cytoplasm is
the fluid content inside the plasma membrane. It also contains many specialised
cell organelles. Each of these organelles performs a specific function for the
cell.
5. Cell Organelles: Every cell has a membrane around it
to keep its content separate from the external environment. The different
components of cell perform different function and these components are called
cell organelles.
(i) Endoplasmic
Reticulum (ER) (Channels, Network for transport):
The ER is a large network of membrane-bound tubes and sheets. It looks like long tubules or round or oblong bags.
There are two types of ER-Rough endoplasmic reticulum [RER] and smooth endoplasmic reticulum [SER]. RER has particles called ribosomes attached to its surface. The ribosomes Endoplasmic Reticulum are the sites of protein manufacture.
The SER helps in the
manufacture of fat molecules, or lipids, important for cell function. Some of
these proteins and lipids help in building the cell membrane. This process is
known as membrane biogenesis. Some other proteins and lipids function as
enzymes and hormones.
DR OMAR CLASSES BARRA MOB-9450149685
The one function of ER is to serve as channels for the transport of materials between various regions of the cytoplasm or between the cytoplasm and the nucleus. The ER also functions as a cytoplasmic framework providing a surface for some of the biochemical activities of the cell.
(ii) Golgi Apparatus (Packaging): The golgi
apparatus, first described by Camillo Golgi, consists of a system of
membrane-bound vesicles arranged approximately, parallel to each other in
stacks called cisterns.
The material synthesised near the ER is packaged and dispatched to various
targets inside and outside the cell through the Golgi apparatus. It’s function
include the storage, modification and packages of products in vesicles. In some
cases complex sugar may be made from simple sugar in the Golgi apparatus. It is
also involved in the formation of lysosomes.
(iii) Lysosomes [Suichge
bags] (Cleanliness of cell): Lysosomes are a kind of waste dispatch and disposal system
of the cell. Lysosome help to keep the cell clean by digesting any foreign
material as well as worn-out cell organelles. Foreign materials entering the
cells such as bacteria or food, as well as old organelles, end up in the
lysosome, which break them up into small pieces. They are able to do this
because they contain powerful digestive enzymes capable of breaking down all
organic material. Under abnormal condition, when the cell gets damaged,
lysosomes may burst and the enzymes digest their own cell. Therefore they are
also known as “suicide bags”
(iv) Mitochondria (Powerhouse,
Energy provider): Mitochondria are known as powerhouses of the cell. The
energy required for various chemical activities needed for life is released by
mitochondria in the form of ATP [Adenosine Triphosphate] molecules. ATP is
known as energy currency of the cell. Mitochondria have two membrane coverings
instead of just one. The outer membrane is very porous while the inner membrane
is deeply folded. They are able to make some of their own protein.
(v) Plastids: Plastids are
present only in plant cells. There are two types of plastids chromoplasts and
leucoplasts. Chromoplasts are the coloured plastids present in leaves, flowers
and fruits. Plastids containing the pigment chlorophyll are known as
chloroplasts. They are important for photosynthesis in plants. Chloroplasts
also contain various yellow or orange pigments in addition to chlorophyll.
Leucoplasts are found primarily in organelles in which materials such as
starch, oils and protein granules are stored.
The internal
organisation of the plastids consists of numerous membrane layers embedded in a
material called stroma. Plastids are similar to mitochondria in external
structure. Plastids have their own DNA and ribosomes.
(vi) Vacuoles (Storage): Vacuoles are
storage sacs for solid or liquid contents. Vacuoles are small-sized in animal
cells while plant cells have very large vacuoles [50% to 90% cell volume].
In plant cells, vacuoles
are full of cell sap and provide turgidity and rigidity to the cell. In Amoeba,
the food’vacuole contain the food items that is consumed it and contractile
vacuoles expels excess water and some wastes from the cell.
DR OMAR CLASSES BARRA
MOB-9450149685
Matter in Our
Surroundings Class 9 Notes Understanding the Lesson
1.
Matter is everything around you. Everything in this universe is made up
of material which in scientific term is called matter.
2.
Matter can be defined as anything that occupies space possesses mass,
offers resistance and can be felt by one or more of our senses.
3.
Examples: Water, air, plant, animal, stones, clouds, etc.
Matter is classified on the basis of their physical and chemical nature.
·
Physical
classification: On the basis of physical properties, matter has been classified
as solid, liquid and gas.
·
Chemical
classification: On the basis of chemical composition, matter has been
classified as element, compound and mixture.
4.
Properties of Matter
·
Matter
is made up of small particles.
·
Particles
of matter have space between them.
·
Particles
of matter are continuously moving.
·
Particles
of matter attract each other because of force of attraction.
5. States of Matter
This classification is done on the basis of arrangement among particles, energy
of particles and the distance between the particles.
(i) Solids:
·
They
have fixed shape and definite volume.
·
They
have small interparticle distances.
·
They
are incompressible.
·
They
are rigid.
·
They
have high density and do not diffuse.
·
They
have strong intermolecular forces of attraction.
·
Their
constituent particles are very closely packed.
·
Their
kinetic energy is very less.
Examples: Sugar, salt, etc.
(ii) Liquids:
·
They
do not have fixed shape but have fixed volume.
·
Their
interparticle distances are larger than solids.
·
They
are almost incompressible.
·
They
have low density than solids.
·
Their
interparticle forces of attraction are weaker than solids.
·
Their
constituent particles are less closely packed.
·
They
assume the shape of the portion of the container they occupy.
·
They
can flow and thus can be called fluids.
·
The
kinetic energy of particles is more than that of solids.
·
Examples:
Milk, water, etc.
(iii)
Gases:
·
They
have neither fixed shape nor fixed volume.
·
Their
interparticle distances are largest among the three states of matter.
·
They
have high compressibility.
·
They
have least density and diffuse.
·
Their
interparticle forces of attraction are weakest.
·
Their
constituent particles are free to move about.
·
They
can expand to occupy larger volume.
·
They
are also called vapour.
·
The
particles of gases have maximum kinetic energy.
·
Examples:
H2, N2, CO2 etc.
6.
Interchange of States of Matter
·
Matter
can be changed from one state to another state.
·
Most
of the metals, which are solid change into liquid on heating and then into
vapour on further heating.
·
The
change of state of matter depends on:
(i) Temperature
(ii) Pressure
7.
Effect of Change of Temperature
8.
The temperature effect on heating a solid varies depending on the nature of the
solid and the conditions required for bringing the change.
9.
Generally on heating, temperature of substances increases. But during state
transformation, temperature remains same.
10.
On heating: The kinetic energy of particles increases which overcomes the force
of attraction between the particles thereby solid melts and is converted to a
liquid.
11. Melting point: It is the temperature at which a solid changes to a liquid at
atomospheric pressure.
12.
Different substances have different melting points.
13.
Higher the melting point means large forces of attraction between the
particles.
14.
Melting point of ice is 273.16 K.
15.
The process of melting is also known as fusion.
16.
Melting point is characteristic propertyof a substance.
17.
Latent heat: The hidden heat which breaks the force of attraction between the
molecule is called latent heat.
18.
It is the heat supplied to a substance during the change of its state.
19.
It is the heat energy hidden in the bulk of matter.
20.
Latent heat of fusion: Amount of heat energy required to convert 1 kg of a
solid into a liquid at atmospheric pressure at its melting point is known as
latent heat of fusion of a substance.
21.
A solid having stronger interparticle forces has greater latent heat of fusion.
22.
Latent heat of fusion of water is 333.7 kJ/kg.
23.
Boiling point: The temperature at which a liquid starts boiling at
atmospheric pressure is known as its boiling point.
24.
A liquid having weaker interparticle forces has lower boiling point and is more
volatile.
25.
Latent heat of vapourisation: The amount of heat energy required to convert
1 kg of a liquid into a gas at atmospheric pressure at its boiling point is
known as latent heat of vapourisation of the substance.
26.
Latent heat of vapourisation of water is 2259 kJ/kg. Thus 1 kg of water in the
form of steam at 373 K has 2259 kJ more energy than 1 kg of water at 373 K.
27.
Condensation: The change of state from gas to liquid is called
condensation.
28.
The condensation process is reverse of vapourisation.
29.
Freezing: The change of state from liquid to solid is called freezing.
30.
Freezing is the reverse of melting or fusion.
31.
Sublimation: Sublimation involves direct conversion of a solid into the
gaseous state on heating and vice-versa.
32.
Dry ice sublimes at -78 °C (195 K).
33.
Camphor, ammonium chloride, iodine and naphthalene are some substances which
undergo sublimation.
34.
Effect of Change of Pressure
In
the gaseous state, the interparticle spaces are very large and attractive
forces between the particles are negligible. Because of large interparticle
space, gases are highly compressible. When pressure is applied on a gas,
enclosed in a cylinder, its molecules move closer and the gas undergoes
appreciable compression. As the molecules move closer, the attractive forces
between the molecules increase. At a sufficiently high pressure, the gas
changes into liquid.
(i)
Solid CO2 is stored under high pressure. At a pressure of 1
atmosphere, solid CO2 changes directly into gas without passing through the
liquid state. Solid CO2 is known as dry ice. Thus, we can
conclude that we can liquefy gas by applying pressure and reducing temperature.
(ii)
Change in volume from gaseous state to liquid state is very large whereas
change in volume from liquid state to solid state is very small (negligible).
This is due to the reason that in liquid the interparticle spaces are very
small in a liquid.
(iii)
Atmospheric pressure: The pressure exerted by the atmosphere or air is called
atmospheric pressure. It decreases with increase in height.
(iv)
Atmosphere (atm) is a unit of pressure.
(v)
The SI unit of pressure is pascal (pa).
1 atm = 1.01 x 105pa
1 bar = 1 x 105 pa 1
bar = 1.01 atm.
(vi)
Evaporation: The phenomenon of change of a liquid into vapour at any
temperature below its boiling point is called evaporation.
(vii)
Particles of matter possess kinetic energy. At a particular temperature, in a
sample of liquid all the particles do not have same kinetic energy. There is a
small fraction of molecule which has enough kinetic energy to overcome the
attractive forces of other particles. If such a particle happens to come near
the surface, it escapes into vapour state and evaporation takes place.
35.
Factors Affecting the Rate of evaporation
(i)
Surface area: Evaporation is a surface phenomenon, i.e., only the particles on
the surface of the liquid gets converted into vapour. Thus, greater is the
surface area, more is the rate of evaporation. For example, clothes dry faster
when they are well spread out.
(ii)
Increase in temperature: The rate of evaporation increases with increase in
temperature. At higher temperature greater number of particles have enough
kinetic energy to escape into the vapour state. For example, clothes dry faster
in summer than in winter.
(iii)
Decrease in humidity: The amount of water vapour present in air is referred to
as humidity. The air cannot hold more than a definite amount of water vapour at
a given temperature. If the humidity is more, the rate of evaporation
decreases. For example, clothes do not dry easily during rainy season because
the rate of evaporation is less due to high moisture content in the air.
(iv) Increase in the
speed of the wind: With the increase in wind speed, the particles of water vapour
move away with the wind, decreasing the amount of water vapour in the
surrounding. For example, wet clothes dry faster on a windy day.
(v) Nature of liquid:
Different liquids have different rates of evaporation. A liquid having weaker
interparticle attractive forces evaporates at a faster rate because less energy
is required to overcome the attractive forces. For example, acetone evaporates
faster than water.
(vi) Evaporation causes
cooling: Only the liquid particles having high kinetic energy leave the surface
of the liquid and get converted into vapour. As a result, the average kinetic
energy of the remaining particles of the liquid decreases and hence the
temperature falls. Thus, evaporation causes cooling.
Class
9 Science Chapter 1 Important Definitions
Melting Point: It is the
temperature at which a solid changes into liquid at atmospheric pressure.
Freezing point: The temperature at
which a liquid freezes to become a solid at atmospheric pressure is called the
freezing point.
Boiling point: The temperature at
which a liquid starts boiling at atmospheric pressure is called its boiling
point.
Latent heat of
vapourisation: The amount of heat energy that is required to change 1 kg
of liquid into vapour at atmospheric pressure at its boiling point is called
latent heat of vapourisation.
Condensation: The process of
changing a gas (or vapour) to a liquid by cooling is called condensation.
Sublimation: Sublimation involves direct conversion of a solid into the gaseous
state on heating and vice-versa.
Latent heat: The hidden heat
which breaks the force of attraction between the molecules is known as latent
heat.
Latent heat of fusion: The heat of energy
required to convert 1 kg of a solid into liquid at atmospheric pressure, as its
melting point, is known as latent heat of fusion.
Boiling: Boiling is a bulk
phenomenon. Particles from the bulk (whole) of the liquid change into vapour
state.
Evaporation: Evaporation is a
surface phenomenon. Particles from the surface gain enough energy to overcome
the force of attraction present in the liquid and change into vapour state.
States of Matter
- Matter can be classified as solid, liquid and gas on the basis of interparticle forces and the arrangement of particles.
- These three forms of matter are interconvertible by increasing or decreasing pressure and temperature. For example, ice can be converted from solid to a liquid by increasing the temperature.
Property Solid Liquid Gas Shape and volume Fixed shape and volume No fixed shape but has volume Neither definite shape nor volume Energy Lowest Medium Highest Compressibility Difficult Nearly difficult Easy Arrangement of molecules Regular and closely arranged Random and little sparsely arranged Random and more sparsely arranged Fluidity Cannot flow Flows from higher to lower level Flows in all directions Movement Negligible Depends on interparticle attraction Free, constant and random Interparticle space Very less More Large Interparticle attraction Maximum Medium Minimum Density Maximum Medium Minimum Rate of diffusion Negligible It depends on interparticle attraction. Maximum
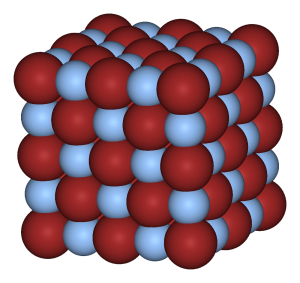
Atomic view of the three states of matter


Evaporation
The phenomenon by which molecules in liquid state undergo a spontaneous transition to the gaseous phase at any temperature below its boiling point is called evaporation.
- For example, the gradual drying of damp clothes is caused by the evaporation of water to water vapour.
Factors affecting evaporation
- Temperature: The rate of evaporation increases with an increase in temperature.
- Surface area: The rate of evaporation increases with an increase in surface area.
- Humidity: The rate of evaporation decreases with an increase in humidity.
- Wind speed: The rate of evaporation increases with an increase in wind speed.
Cooling due to evaporation
During evaporation, the particles of a liquid absorb energy from the surroundings to overcome the inter-particle forces of attraction and undergo the phase change. The absorption of heat from the surrounding makes the surrounding cool.
For example, sweating cools down our body.Physical Nature of Matter
- A physical property is that aspect of the matter that can be observed or measured without changing its nature or composition.
- It is independent of the amount of matter present.
- Physical properties include appearance, colour, odour, density, texture, melting point, boiling point, solubility, etc.
Characteristics of Particles of Matter
Matter
Matter is anything that has mass and occupies space.
- Everything that we can touch, see, hear, taste and also smell is matter.
- It is made up of really tiny particles which cannot be seen through the eye.
The particles of which the matter is comprised influence its state and properties (physical and chemical).
1. Particles of matter have spaces between them
- This characteristic is one of the concepts behind the solubility of a substance in other substances. For example, on dissolving sugar in water, there is no rise in water level because the particles of sugar get into the interparticle spaces between the water particles.
2. Particles of matter are always in motion
- Particles of the matter show continuous random movements due to the kinetic energy they possess.
- A rise in temperature increases the kinetic energy of the particles, making them move more vigorously.
3. Particles of matter attract each other
In every substance, there is an interparticle force of attraction acting between the particles. To break a substance we need to overcome this force. The strength of the force differs from one substance to another.Diffusion
When the particles of matter intermix on their own with each other, the phenomenon is called diffusion. For example, spreading of ink in water.
- During diffusion, the particles occupy the interparticle spaces.
- The rate of diffusion increases with increase in the temperature, due to increase in kinetic energy of the particles.
Can Matter Change Its State?
Effect of change of temperature on state of matter
On increasing temperature, the kinetic energy of the particles of the matter increases and they begin to vibrate with a higher energy. Therefore, the interparticle force of attraction between the particles reduces and particles get detached from their position and begin to move freely.
- As a result, the state of matter begins to change.
- Solids undergo a phase change to form liquids.
- Similarly, liquids also undergo a phase change to form gases.
Melting point
The melting point of a solid is defined as the temperature at which solid melts to become liquid at the atmospheric pressure.
- At melting point, these two phases, i.e., solid and liquid are in equilibrium, i.e., at this point both solid state and liquid state exist simultaneously.
Boiling point
The boiling point of a liquid is defined as the temperature at which the vapour pressure of the liquid is equal to the atmospheric pressure.
Latent heat of fusion
It is the amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point.
Latent heat of vaporisation
It is the amount of heat energy that is required to change 1 kg of a liquid into gas at atmospheric pressure at its boiling point.
Sublimation
The transition of a substance directly from its solid phase to gaseous phase without changing into the liquid phase (or vice versa) is called sublimation.

Sublimation – Solid to Gas Phase Transformation Effect of change in pressure on state of matter
By applying pressure, the interparticle spaces between particles of matter decreases. Thus, by applying pressure and reducing temperature we can convert a solid to liquid and a liquid to gas.
Flowchart for inter-conversion of the three states of matter

Frequently asked Questions on CBSE Class 9 Biology Notes Chapter 1: Matter in Our Surroundings
What is ‘Latent heat of fusion’?
The latent heat of fusion is the enthalpy change of any amount of substance when it melts.
What does ‘Sublimation critical point’ mean?
Sublimation critical point refers to the maximum or minimum temperature and pressure beyond which the state of the matter cannot be changed.
What does ‘Interconversion of matter’ mean?
Interconversion of matter refers to the change of one state to another. It is a process by which matter changes from one state to another and back to its original state, without any change in its chemical composition.
DR OMAR CLASSES BARRA KANPUR
DR OMAR CLASSES, BARRA-3, MOBILE-9450149685
Introduction to Force
A force
is an effort that changes the state of an object at rest or at motion. It can
change an object’s direction and velocity. Force can also change the shape of
an object.
Effects of Force
Some
effects of force include the following:
- Force
moves stationary objects
- Force
stops objects from moving
- Force
changes the shape of a body
- Force
changes the direction of motion
Push is
defined as an action of force which causes an object to move from its place.
The following are the examples of push:
- Opening
and closing of the door.
- Pushing
the table.
- Pushing
a car.
- Pushing
of the thumb pins.
- Walking
Pull is
defined as an action to make move by either tugging or dragging. The following
are the examples of pull:
- Plucking
the string of a guitar.
- Pulling
ropes while playing tug of war.
- Opening
the drawer.
- Pulling
the window curtain.
- Opening
and closing of the doors.
Balanced and Unbalanced Forces
When
balanced forces are applied to an object, there will be no net effective
force acting on the object. Balanced forces do not cause a change in
motion.
Unbalanced
forces acting on an object change its speed and/or direction of motion. It
moves in the direction of the force with the highest magnitude.
Net force
When
multiple forces act on a body, they can be resolved into one component known as
the net force acting on the object. The net force decides the direction of
motion.
DR OMAR CLASSES, BARRA-3, MOBILE-9450149685
Frictional force
The
force that opposes relative motion is called friction. It arises between the
surfaces in contact.
Example:
When we try to push a table and it does not move is because it is balanced by
the frictional force.
First Law of Motion
A body
continues to be in the state of rest or uniform motion in a straight line
unless acted upon by an external unbalanced force. The First Law is also called
the Law of Inertia.
Inertia
Basically,
all objects have a tendency to resist the change in the state of motion or
rest. This tendency is called inertia. All bodies do not have the same inertia.
Inertia depends on the mass of a body. Mass of an object is the measure of its
inertia.
More
the mass → more inertia and vice versa.
Inertia of Rest
An
object stays at rest, and it remains at rest until an external force affects
it. Example: When a car accelerates, passengers may feel as though their bodies
are moving backward. In reality, inertia is making their bodies stay in place
as the car moves forward.
Inertia of Motion
An
object will continue to be in motion until a force acts on it. Example: A
hockey puck will continue to slide across the ice until acted upon by an
outside force.
DR OMAR CLASSES, BARRA-3, MOBILE-9450149685
Second Law of Motion
In
order to understand second law, we need to first understand momentum.
Momentum
Impacts
produced by objects depend on their mass and velocity. The momentum of an
object is defined as the product of its mass and velocity. p = mv. Vector
quantity, has direction and magnitude. Some examples of momentum include: A
baseball flying through the air and a bullet fired from a gun.
Second Law of Motion
The
rate of change of momentum of an object is directly proportional to the applied
unbalanced force in the direction of the force.
\(\begin{array}{l}\frac{\Delta p}{t}\alpha
\frac{m(v-u)}{t}\end{array} \)
Here, a
[ = (v – u)/t ] is the acceleration, which is the rate of change in velocity.
\(\begin{array}{l}\frac{\Delta p}{t}\alpha ma\end{array} \)
\(\begin{array}{l}F \alpha ma\end{array} \)
F = kma
For 1
unit of force on 1 kg mass with the acceleration of 1m/s2, the value of k = 1.
Therefore,
F = ma.
Conservation of Momentum
Concept of system
- The
part of the universe chosen for analysis is called a system.
- Everything
outside the system is called an environment.
- For
example, a car moving with constant velocity can be considered a system.
All the forces within the car are internal forces and all forces acting on
the car from the environment are external forces like friction.
Conservation of momentum
- The
total momentum of an isolated system is conserved.
- Isolated
system → net external force on the
system is zero.
- Example:
Collision of 2 balls A and B.
From
Newtons 3rd law F_{AB} = -F_{BA}
\(\begin{array}{l}m_{A}\frac{V_{a}-U_{a}}{t} =
m_{B}\frac{V_{b}-U_{b}}{t}\end{array} \)
\(\begin{array}{l}m_{A}U_{A} + m_{B}U_{B} = m_{A}V_{A} +
m_{B}V_{B}\end{array} \)
Third Law of Motion
Newton’s
3rd law states that every action has an equal and opposite reaction. Action and
reaction forces are equal, opposite and acting on different bodies.
Inertial and Non-inertial frames
- A
non-inertial frame of reference is a frame of reference in which Newton’s
laws of motion do not hold. A non-inertial reference frame is a frame of
reference that is undergoing acceleration with respect to an inertial
frame. An accelerometer at rest in a non-inertial frame will, in general,
detect a non-zero acceleration.
- A
frame of reference where Newton’s Laws hold is known as an inertial frame
of reference.
DR OMAR CLASSES, BARRA-3, MOBILE-9450149685

















No comments:
Post a Comment